��Ŀ����
��2007?��ͷ��ģ����1��������ͪ��һ���кɻ���ζ����ǿ�������õĻ�ѧ�Լ������Ľṹ��ʽ��ͼ��ʾ��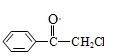 ��������ͪ�����ܾ��еĻ�ѧ������
��������ͪ�����ܾ��еĻ�ѧ������
a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ
d��ˮ�ⷴӦ e��������Ӧ
��2��������
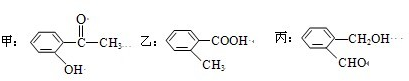
����д�����к��������ŵ����ƣ�
�����б�������Щ�����ﻥΪͬ���칹�壺
���밴������ǿ�������мס��ҡ�����˳��
��3���ɱ�ϩ�����з�Ӧ���Ƶ�F��G���ָ߷��ӻ�������Ƕ��dz��õ����ϣ�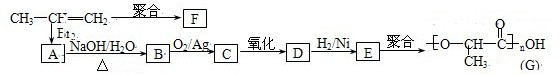
�پۺ���F�Ľṹ��ʽ��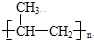
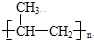 ��
��
��Bת��ΪC�Ļ�ѧ����ʽ��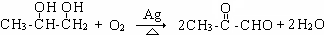
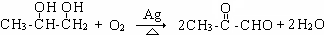 ��
��
����һ�������£�������E����ȥ������ˮ�γ�һ����Ԫ��״������û�����Ľṹ��ʽ��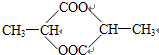
 ��
��
 ��������ͪ�����ܾ��еĻ�ѧ������
��������ͪ�����ܾ��еĻ�ѧ������c��e
c��e
������ĸ��ţ���a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ
d��ˮ�ⷴӦ e��������Ӧ
��2��������

����д�����к��������ŵ����ƣ�
ȩ�����ǻ�
ȩ�����ǻ�
�������б�������Щ�����ﻥΪͬ���칹�壺
�ס��ҡ���
�ס��ҡ���
�����밴������ǿ�������мס��ҡ�����˳��
�ң��ף���
�ң��ף���
����3���ɱ�ϩ�����з�Ӧ���Ƶ�F��G���ָ߷��ӻ�������Ƕ��dz��õ����ϣ�

�پۺ���F�Ľṹ��ʽ��
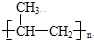
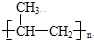
��Bת��ΪC�Ļ�ѧ����ʽ��
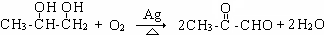
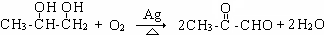
����һ�������£�������E����ȥ������ˮ�γ�һ����Ԫ��״������û�����Ľṹ��ʽ��


��������1�������л�������ŵ������ƶ��л�����ܾ��е����ʣ�
��2�������л���Ľṹ��ʽ���жϺ��������ţ����ͬ���칹��Ķ����жϣ��л�����-COOH���Դ��ڷ��ǻ������ǻ���ȩ�������������ԣ�
��3��CH3CH=CH2���巢���ӳ�����AΪCH3CHBrCH2Br��ˮ������BΪCH3CHOHCH2OH���ڴ��������±���������CΪ ����DΪ
����DΪ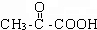 ��EΪCH3CHOHCOOH��CH3CH=CH2�ɷ����Ӿ۷�Ӧ������FΪ
��EΪCH3CHOHCOOH��CH3CH=CH2�ɷ����Ӿ۷�Ӧ������FΪ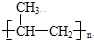 ���Դ˽����⣮
���Դ˽����⣮
��2�������л���Ľṹ��ʽ���жϺ��������ţ����ͬ���칹��Ķ����жϣ��л�����-COOH���Դ��ڷ��ǻ������ǻ���ȩ�������������ԣ�
��3��CH3CH=CH2���巢���ӳ�����AΪCH3CHBrCH2Br��ˮ������BΪCH3CHOHCH2OH���ڴ��������±���������CΪ
 ����DΪ
����DΪ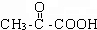 ��EΪCH3CHOHCOOH��CH3CH=CH2�ɷ����Ӿ۷�Ӧ������FΪ
��EΪCH3CHOHCOOH��CH3CH=CH2�ɷ����Ӿ۷�Ӧ������FΪ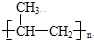 ���Դ˽����⣮
���Դ˽����⣮����⣺��1�����л��ﺬ���ʻ����ɷ����ӳɷ�Ӧ������-Cl���ɷ���ȡ����ˮ�ⷴӦ�����ܷ�����ȥ��������Ӧ��
�ʴ�Ϊ��c��e��
��2���ٱ��к���ȩ�����ǻ��ȹ����ţ��ʴ�Ϊ��ȩ�����ǻ���
�ڼס��ҡ����������ʵķ���ʽ��ͬ�����ṹ��ͬ������ͬ���칹�壬�ʴ�Ϊ���ס��ҡ�����
���л�����-COOH���Դ��ڷ��ǻ������ǻ���ȩ�������������ԣ�������˳��Ϊ�ң��ף�����
�ʴ�Ϊ���ң��ף�����
��3��CH3CH=CH2���巢���ӳ�����AΪCH3CHBrCH2Br��ˮ������BΪCH3CHOHCH2OH���ڴ��������±���������CΪ ����DΪ
����DΪ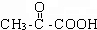 ��EΪCH3CHOHCOOH��CH3CH=CH2�ɷ����Ӿ۷�Ӧ������FΪ
��EΪCH3CHOHCOOH��CH3CH=CH2�ɷ����Ӿ۷�Ӧ������FΪ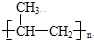 ��
��
�������Ϸ�����֪FΪ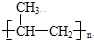 ���ʴ�Ϊ��
���ʴ�Ϊ��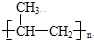 ��
��
��BΪCH3CHOHCH2OH���ڴ��������±���������CΪ ����Ӧ�ķ���ʽΪ
����Ӧ�ķ���ʽΪ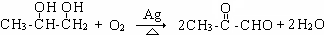 ��
��
�ʴ�Ϊ��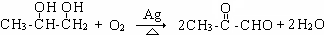 ��
��
��EΪCH3CHOHCOOH������-COOH��-OH��������E����ȥ������ˮ�γ�һ����Ԫ��״�����Ϊ������Ӧ��������Ϊ ��
��
�ʴ�Ϊ�� ��
��
�ʴ�Ϊ��c��e��
��2���ٱ��к���ȩ�����ǻ��ȹ����ţ��ʴ�Ϊ��ȩ�����ǻ���
�ڼס��ҡ����������ʵķ���ʽ��ͬ�����ṹ��ͬ������ͬ���칹�壬�ʴ�Ϊ���ס��ҡ�����
���л�����-COOH���Դ��ڷ��ǻ������ǻ���ȩ�������������ԣ�������˳��Ϊ�ң��ף�����
�ʴ�Ϊ���ң��ף�����
��3��CH3CH=CH2���巢���ӳ�����AΪCH3CHBrCH2Br��ˮ������BΪCH3CHOHCH2OH���ڴ��������±���������CΪ
 ����DΪ
����DΪ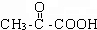 ��EΪCH3CHOHCOOH��CH3CH=CH2�ɷ����Ӿ۷�Ӧ������FΪ
��EΪCH3CHOHCOOH��CH3CH=CH2�ɷ����Ӿ۷�Ӧ������FΪ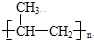 ��
���������Ϸ�����֪FΪ
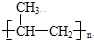 ���ʴ�Ϊ��
���ʴ�Ϊ��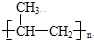 ��
�� ��BΪCH3CHOHCH2OH���ڴ��������±���������CΪ
 ����Ӧ�ķ���ʽΪ
����Ӧ�ķ���ʽΪ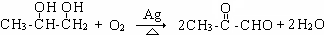 ��
���ʴ�Ϊ��
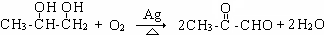 ��
�� ��EΪCH3CHOHCOOH������-COOH��-OH��������E����ȥ������ˮ�γ�һ����Ԫ��״�����Ϊ������Ӧ��������Ϊ
 ��
���ʴ�Ϊ��
 ��
�����������⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ�����ע������л���Ľṹ�����ʣ�ע��ͬ���칹����жϣ��״���Ϊ��3����ע���Ա�ϩ�����ʽ�Ϸ�Ӧ���������ƶϣ����չ����ŵ�����Ϊ������Ĺؼ���

��ϰ��ϵ�д�
 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�
�����Ŀ
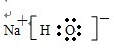

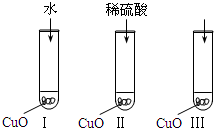 ��2007?��ͷ��ģ������ͭ��һ�ֺ�ɫ���壬������ϡ���ᣮijͬѧ��֪����ϡ������������ӣ�H2O��H+��SO42-����ʹ����ͭ�ܽ⣮�������һ��ͨ����ͼ��͢�����ʵ��������̽�����
��2007?��ͷ��ģ������ͭ��һ�ֺ�ɫ���壬������ϡ���ᣮijͬѧ��֪����ϡ������������ӣ�H2O��H+��SO42-����ʹ����ͭ�ܽ⣮�������һ��ͨ����ͼ��͢�����ʵ��������̽����� ��2007?��ͷ��ģ��2012��6��16���ҹ��ɹ������ˡ����ݾźš������־���й��˵�̫��ʱ����ǰ����һ�����䡰��š�ʱ���£�N2H4����Ϊ�����������ȼ�ϣ�NO2Ϊ����������Ӧ����N2��ˮ��������֪��
��2007?��ͷ��ģ��2012��6��16���ҹ��ɹ������ˡ����ݾźš������־���й��˵�̫��ʱ����ǰ����һ�����䡰��š�ʱ���£�N2H4����Ϊ�����������ȼ�ϣ�NO2Ϊ����������Ӧ����N2��ˮ��������֪��