题目内容
 (2007?汕头二模)2012年6月16日我国成功发射了“神州九号”.这标志着中国人的太空时代又前进了一大步.发射“神九”时用肼(N2H4)作为火箭发动机的燃料,NO2为氧化剂,反应生成N2和水蒸气.已知:
(2007?汕头二模)2012年6月16日我国成功发射了“神州九号”.这标志着中国人的太空时代又前进了一大步.发射“神九”时用肼(N2H4)作为火箭发动机的燃料,NO2为氧化剂,反应生成N2和水蒸气.已知:N2(g)+2O2(g)=2NO2(g);△H=+67.7kJ/mol
N2H4(g)+O2(g)=N2(g)+2H2O(g);△H=-534kJ/mol
下列关于肼和NO2反应的热化学方程式中,正确的是( )
分析:根据盖斯定律,由已知热化学方程式乘以合适的系数进行加减构造目标热化学方程式,反应热也乘以相应的系数进行相应的运算.
解答:解:已知:①N2(g)+2O2(g)=2NO2(g);△H=+67.7kJ/mol
②N2H4(g)+O2(g)=N2(g)+2H2O(g);△H=-534kJ/mol
根据盖斯定律,②×2-①得2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g);故△H=2×(-534kJ/)-67.7kJ/mol=-1135.7 kJ/mol,
肼和NO2生成N2和水蒸气的热化学方程式为:2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g);△H=-1135.7 kJ/mol,
A、水的状态应为气态,不是液态,故A错误;
B、反应热为△H=-1135.7 kJ/mol,不是△H=-1000.3 kJ/mol,故B错误;
C、1molN2H4(g)反应,水的状态应为气态,不是液态,反应热为△H=-567.86 kJ/mol,故C错误;
D、由上述分析可知,肼和NO2生成N2和水蒸气的热化学方程式为:2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g);△H=-1135.7 kJ/mol,故D正确;
故选D.
②N2H4(g)+O2(g)=N2(g)+2H2O(g);△H=-534kJ/mol
根据盖斯定律,②×2-①得2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g);故△H=2×(-534kJ/)-67.7kJ/mol=-1135.7 kJ/mol,
肼和NO2生成N2和水蒸气的热化学方程式为:2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g);△H=-1135.7 kJ/mol,
A、水的状态应为气态,不是液态,故A错误;
B、反应热为△H=-1135.7 kJ/mol,不是△H=-1000.3 kJ/mol,故B错误;
C、1molN2H4(g)反应,水的状态应为气态,不是液态,反应热为△H=-567.86 kJ/mol,故C错误;
D、由上述分析可知,肼和NO2生成N2和水蒸气的热化学方程式为:2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g);△H=-1135.7 kJ/mol,故D正确;
故选D.
点评:本题考查热化学方程式的书写,难度不大,注意书写规则与盖斯定律的利用.

练习册系列答案
 口算心算速算应用题系列答案
口算心算速算应用题系列答案 同步拓展阅读系列答案
同步拓展阅读系列答案
相关题目



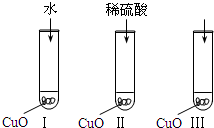 (2007?汕头二模)氧化铜是一种黑色固体,可溶于稀硫酸.某同学想知道是稀硫酸的哪种粒子(H2O,H+,SO42-)能使氧化铜溶解.请你和他一起通过如图Ⅰ、Ⅱ和Ⅲ三个实验完成这次探究活动.
(2007?汕头二模)氧化铜是一种黑色固体,可溶于稀硫酸.某同学想知道是稀硫酸的哪种粒子(H2O,H+,SO42-)能使氧化铜溶解.请你和他一起通过如图Ⅰ、Ⅱ和Ⅲ三个实验完成这次探究活动. ,则苯氯乙酮不可能具有的化学性质是
,则苯氯乙酮不可能具有的化学性质是



