��Ŀ����
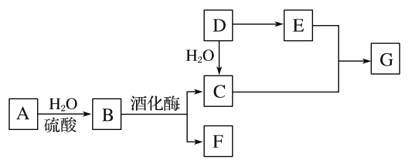
����Ŀ����������һ����ȻȾ�ϣ���ҵ�Ͽ���ʯ�͵��ѽ����ͨ����ͼ��Ӧ�Ƶã�
��֪��![]()
![]() CH3CHO+
CH3CHO+![]()
![]()
![]() +H2O
+H2O
��ش��������⣺
![]() �Լ�XΪ ______ ��
�Լ�XΪ ______ ��
![]() ���������Na��NaOH��
���������Na��NaOH��![]() �����ʵ����ֱ�Ϊ3mo1��2mol��1mol����E�Ľṹ��ʽΪ ______ ��
�����ʵ����ֱ�Ϊ3mo1��2mol��1mol����E�Ľṹ��ʽΪ ______ ��
![]() �������еĺ��������ų�������
�������еĺ��������ų�������![]() ��� ______
��� ______ ![]() ���
���![]() ��
��
![]() ��Ӧ
��Ӧ![]() �Ļ�ѧ����ʽΪ ______ ���䷴Ӧ������ ______ ��
�Ļ�ѧ����ʽΪ ______ ���䷴Ӧ������ ______ ��
![]() ������������G��ͬ���칹�干�� ______ �֣����к˴Ź�����������5��壬�������Ϊ2:2:2:1:1���� ______ ��
������������G��ͬ���칹�干�� ______ �֣����к˴Ź�����������5��壬�������Ϊ2:2:2:1:1���� ______ ��
�����ڷ������� �ڱ�����������ȡ���� ������![]() ��Һ������ɫ��Ӧ
��Һ������ɫ��Ӧ
![]() �����ȡ�����صķ���Ҳ�ܺϳ����ȩ(
�����ȡ�����صķ���Ҳ�ܺϳ����ȩ(![]() )��д���Ʊ����ȩ�����л���Ľṹ��ʽ ______ ��
)��д���Ʊ����ȩ�����л���Ľṹ��ʽ ______ ��
���𰸡�NaOH��ˮ��Һ 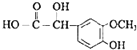 �ǻ����ʻ�
�ǻ����ʻ� ![]() ������Ӧ 9
������Ӧ 9 ![]()
![]() ��
��![]()
��������
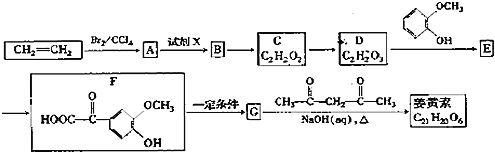
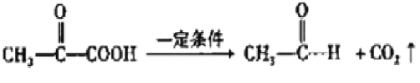
��ϩ���巢���ӳɷ�Ӧ����AΪBrCH2CH2Br����A��D��ϵ��ת����C��D����ʽ��֪��C��ȩ����������D����A����������ˮ��Һ�����������·���ˮ�ⷴӦ����BΪHOCH2CH2OH��B��������������CΪOHC-CHO��C�в���ȩ������������DΪOHC-COOH��1molE���������Na��NaOH��NaHCO3�����ʵ����ֱ�Ϊ3mo1��2mol��1mol�����F�Ľṹ��֪��D��ȩ�������ӳ�����EΪ ��E�е�ȩ������������F��F��һ�������·�����Ϣi�����ȷ�Ӧ����GΪ
��E�е�ȩ������������F��F��һ�������·�����Ϣi�����ȷ�Ӧ����GΪ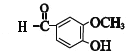 �������Ϣii�������صķ���ʽ����֪�����صĽṹ��ʽΪ��
�������Ϣii�������صķ���ʽ����֪�����صĽṹ��ʽΪ�� ��
��
(1)A��B����±������ˮ�ⷴӦ���Լ�XΪNaOH��ˮ��Һ��
�ʴ�Ϊ��NaOH��ˮ��Һ��
(2)E�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
(3)�����صĽṹ��ʽΪ�� ���������еĺ��������ų���������CH3O����У��ǻ����ʻ���
���������еĺ��������ų���������CH3O����У��ǻ����ʻ���
�ʴ�Ϊ���ǻ����ʻ���
(4)��ӦB��C�Ļ�ѧ����ʽΪ��![]() ������������Ӧ��
������������Ӧ��
�ʴ�Ϊ��![]() ��������Ӧ��
��������Ӧ��
(5)G(
![]() ��ͬ���칹����������������ڷ��������࣬�ڱ�����������ȡ������������FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ������Ա����ϳ����ǻ�֮�⣬��һȡ�������ܵĽṹ��-OOCCH3��-COOCH3��-CH2OOCH�������ڡ��䡢��3�֣����Է�������������ȩ��ͬ���칹�干��9�֣����к˴Ź�����������5��壬�������Ϊ2��2��2��1��1����
��ͬ���칹����������������ڷ��������࣬�ڱ�����������ȡ������������FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ������Ա����ϳ����ǻ�֮�⣬��һȡ�������ܵĽṹ��-OOCCH3��-COOCH3��-CH2OOCH�������ڡ��䡢��3�֣����Է�������������ȩ��ͬ���칹�干��9�֣����к˴Ź�����������5��壬�������Ϊ2��2��2��1��1����![]() ��
��
�ʴ�Ϊ��9��![]() ��
��
(6)�����ȡ�����صķ���Ҳ�ܺϳ����ȩ![]()
![]()
![]() ���Ʊ����ȩ�����л���Ľṹ��ʽΪ
���Ʊ����ȩ�����л���Ľṹ��ʽΪ![]() ��CH3CHO��
��CH3CHO��
�ʴ�Ϊ��![]() ��CH3CHO��
��CH3CHO��

����Ŀ��H2S�ڽ������ӵļ���������ú��������������ҪӦ�á���ش�
��.��ҵ��һ���Ʊ�H2S�ķ������ڴ��������������£�����Ȼ����SO2��Ӧ��ͬʱ���������ܲ������ѭ���������
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ_____________��
��.H2S�����ڼ��ͳ������������ӡ�
(2)H2S�ĵ�һ�����뷽��ʽΪ________��
(3)��֪��25 ��ʱ��Ksp(SnS)��1.0��10��25��Ksp(CdS)��8.0��10��27�����¶��£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��CdCl2��SnCl2�Ļ����Һ��ͨ��H2S����Sn2����ʼ����ʱ����Һ��c(Cd2��)��________(��Һ����仯���Բ���)��
��.H2S��ú����ԭ����������̵���Ҫ�м��塣��Ӧԭ��Ϊ
��.COS(g)��H2(g) ![]() H2S(g)��CO(g)����H����7 kJ��mol��1��
H2S(g)��CO(g)����H����7 kJ��mol��1��
��.CO(g)��H2O(g) ![]() CO2(g)��H2(g)����H����42 kJ��mol��1��
CO2(g)��H2(g)����H����42 kJ��mol��1��
(4)��֪������1 mol�����еĻ�ѧ���������յ����������ʾ��
���� | COS(g) | H2(g) | CO(g) | H2S(g) | H2O(g) | CO2(g) |
����/(kJ��mol��1) | 1 319 | 442 | x | 678 | 930 | 1 606 |
����x��________��
(5)��10 L�ݻ�������ܱ������г���1 mol COS(g)��1 mol H2(g)��1 mol H2O(g)����������������Ӧ��������������ʱ����ϵ��CO��ƽ������������¶�(T)�Ĺ�ϵ��ͼ��ʾ��
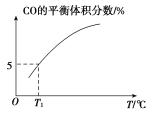
�������¶����ߣ�CO��ƽ���������_____(����������������С��)��ԭ��Ϊ_______
��T1��ʱ�����ƽ��ʱ��ϵ��COS�����ʵ���Ϊ0.80 mol������¶��£�COS��ƽ��ת����Ϊ_____����Ӧ����ƽ�ⳣ��Ϊ_____(������λ��Ч����)��
����Ŀ��X��Y��Z��W����Ԫ�صIJ�����Ϣ���±���ʾ��
Ԫ�� | X | Y | Z | W |
��� ��Ϣ | ������Ԫ�أ�����ϼ�Ϊ+7�� | ��̬ԭ���У�����ռ�ݵ�����ܲ����ΪL������ܼ���ֻ����������������ͬ�ĵ��� | ������ӹ���15���˶�״̬ | ����X�γ����ֳ���������WX2��WX3������WX3��Һ�ܷ�����ɫ��Ӧ |
�ش��������⣺
��1��W�Ļ�̬ԭ�ӵ����Ų�ʽΪ___��X��Y��Z����Ԫ�ص縺���ɴ�С��˳��Ϊ___(�þ����Ԫ�ط�����д)��
��2��������YX4��ZX3��ZX5(��̬��Һ̬ʱ)�У�����ԭ�ӵĹ�����Ͳ���sp3�ӻ�����___ (�ѧʽ����ͬ�������ӹ����������������___��ZX3����___�����Է��ӡ��Ǽ��Է��ӣ���
��3����֪WX3���۵㣺306�棬�е㣺319�棬��WX3�ľ�������Ϊ___��
��4��Zԭ�ӵļ۵��ӹ����ʾʽΪ___��
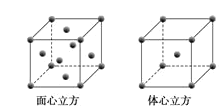
��5��WԪ�صĵ��ʾ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ���ͼ��ʾ������������������Wԭ�ӵ���λ��Ϊ___����W��ԭ�Ӱ뾶Ϊrcm�������ӵ�����ΪNA��������������������ܶȿɱ�ʾΪ___gcm-3��