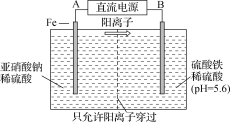
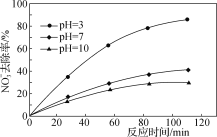
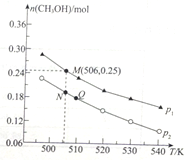
��Ŀ����
����Ŀ����֪��25��ʱ�����ᡢ̼���������ĵ���ƽ�ⳣ�����±���ʾ��
����� | ���� | ̼�� | ������ |
���볣�� | K=1.75��10-5 | K1=4.30��10-7 K2=5.61��10-11 | K1=1.00��10-2 K2=1.02��10-7 |
��1��д��������ĵ�һ������ƽ�ⳣ������ʽ��K1=_____________________��
��2����֪NaHSO3��Һ�����ԣ�д����Һ������Ũ�ȴ�С_____________________��
��3��Na2CO3��Һȥ���۵�ԭ�� _____________________�����û�ѧ�����ʾ��
��4������ͬ�����£��ԱȽϢ�CH3COONa ��NaHCO3 ��Na2SO3ˮ��Һ�ļ���ǿ����������ţ�_____________________��
��5���������¶Ȳ��䣬�ڴ�����Һ�м������������ƣ����������С����_________��������ţ�
A.c(CH3COO-) B.c(H+) C.�������ƽ�ⳣ�� D.����ĵ���̶�
��6��NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kb=_______________ molL-1��
��7��H2SO3��Һ��NaHCO3��Һ��Ӧ����Ҫ���ӷ���ʽΪ_________________________________��
���𰸡�Ka��c(H+)c(HSO3-)/c(H2SO3) c(Na+)>c(HSO3-)>c(H+)��c(SO32-)>c(OH-) CO32-+H2O![]() HCO3-+OH- �ۢڢ� BD 1��10-12 H2SO3+HCO3-��HSO3-+CO2��+H2O
HCO3-+OH- �ۢڢ� BD 1��10-12 H2SO3+HCO3-��HSO3-+CO2��+H2O
��������
��1���������Ƕ�Ԫ���ᣬ��һ�����뷽��ʽΪH2SO3![]() HSO3��+H+�����һ������ƽ�ⳣ������ʽK1= c(H+)c(HSO3��)/c(H2SO3)����2����֪NaHSO3��Һ�����ԣ���˵��HSO3���ĵ���̶ȴ���HSO3����ˮ��̶ȣ�������Һ������Ũ�ȴ�С˳��Ϊc(Na+)>c(HSO3��)>c(H+)��c(SO32��)>c(OH��)����3��Na2CO3��Һ��̼���ˮ�⣬��Һ�Լ��ԣ����Կ���ȥ�����ۣ�ˮ�ⷽ��ʽΪCO32��+H2O
HSO3��+H+�����һ������ƽ�ⳣ������ʽK1= c(H+)c(HSO3��)/c(H2SO3)����2����֪NaHSO3��Һ�����ԣ���˵��HSO3���ĵ���̶ȴ���HSO3����ˮ��̶ȣ�������Һ������Ũ�ȴ�С˳��Ϊc(Na+)>c(HSO3��)>c(H+)��c(SO32��)>c(OH��)����3��Na2CO3��Һ��̼���ˮ�⣬��Һ�Լ��ԣ����Կ���ȥ�����ۣ�ˮ�ⷽ��ʽΪCO32��+H2O![]() HCO3��+OH������4�����ݵ��볣����֪���ԣ����̼����������������Խ������Ӧ�����Խ����ˮ�⣬����Խǿ��������ͬ�����£���CH3COONa��NaHCO3��Na2SO3ˮ��Һ�ļ���ǿ��˳��Ϊ�ۣ��ڣ�������5��A.�ڴ�����Һ�м������������ƣ�c(CH3COO-)����A����B.�ڴ�����Һ�м������������ƣ�c(CH3COO-)�������ƴ�����룬����c(H+)��С��B��ȷ��C.�¶Ȳ��䣬�������ƽ�ⳣ�����䣬C����D.�ڴ�����Һ�м������������ƣ�c(CH3COO-)�������ƴ�����룬����ĵ���̶ȼ�С��D��ȷ����ѡBD����6��NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kb=
HCO3��+OH������4�����ݵ��볣����֪���ԣ����̼����������������Խ������Ӧ�����Խ����ˮ�⣬����Խǿ��������ͬ�����£���CH3COONa��NaHCO3��Na2SO3ˮ��Һ�ļ���ǿ��˳��Ϊ�ۣ��ڣ�������5��A.�ڴ�����Һ�м������������ƣ�c(CH3COO-)����A����B.�ڴ�����Һ�м������������ƣ�c(CH3COO-)�������ƴ�����룬����c(H+)��С��B��ȷ��C.�¶Ȳ��䣬�������ƽ�ⳣ�����䣬C����D.�ڴ�����Һ�м������������ƣ�c(CH3COO-)�������ƴ�����룬����ĵ���̶ȼ�С��D��ȷ����ѡBD����6��NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kb=![]() 1��10-12 molL-1����7�����ԣ������̼��������������H2SO3��Һ��NaHCO3��Һ��Ӧ����Ҫ���ӷ���ʽΪH2SO3+HCO3-��HSO3-+CO2��+H2O��
1��10-12 molL-1����7�����ԣ������̼��������������H2SO3��Һ��NaHCO3��Һ��Ӧ����Ҫ���ӷ���ʽΪH2SO3+HCO3-��HSO3-+CO2��+H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�