��Ŀ����
8����1��25��ʱ����0.1mol•L-1�����ᡡ��0.2mol•L-1�����ᡡ����0.1mol•L-1�Ĵ��ᡡ��0.1mol•L-1�İ�ˮ����0.1mol•L-1��NaOH��Һ����0.1mol•L-1��Ba��OH��2��Һ����pH��С�����˳���Ǣڢ٢ۢܢݢޣ�����ţ���2�����������Т�NaCl����BaSO4 ��H2SO4����KOH ��ʯī����H2CO3����CH3COOH����NH3•H2O �����ǡ���ƾ�����11��SO2����12��Cu ��13��Cl2 ��14��CO2����������ǿ����ʵ��Т٢ڢۢܣ���������������ʵ��Тޢߢࣻ�������ڷǵ���ʵ��Т�⣨11����14����
��3��ijһԪ������Һ��A�����Ԫǿ�ᣨB����pH��ȣ���������Һϡ����ͬ�ı�����pHA��pHB���������=������������������ϡ����Һ�к͵�Ũ�ȵ������NaOH��Һ������ϡ����Һ�����VA��VB�����������=����������
��4��25��ʱ����10���ijǿ����Һ��1�����ijǿ����Һ��Ϻ���Һ��pH=7������ǰ����ǿ����Һ��pH��ǿ����ҺpH֮��Ӧ����Ĺ�ϵ��PH��+PH��=13��
���� ��1�����Ȼ���Ϊǿ����ʣ���ȫ����0.1mol•L-1������������Ũ��Ϊ0.1mol•L-1��������Ϊǿ�����0.2mol•L-1�����ᣬ������Ũ��Ϊ��0.4mol•L-1�����۴���Ϊ������ʣ����ֵ��룬0.1mol•L-1�Ĵ���������Ũ��С��0.1mol•L-1����һˮ�ϰ�Ϊ������ʣ����ֵ��룬0.1mol•L-1�İ�ˮ����������Ũ��С��0.1mol•L-1��������������Ϊǿ����ʣ���ȫ���룬0.1mol•L-1��NaOH��Һ������������Ũ��0.1mol•L-1������������Ϊǿ����ʣ���ȫ���룬0.1mol•L-1��Ba��OH��2��Һ������������Ũ��Ϊ0.2mol•L-1��
��2���������ָ����ˮ��Һ�л�����״̬���ܹ�����Ļ���������ˮ��Һ�л�����״̬���ܹ����磬�������������������������ƶ������ӣ�
�ǵ������ָ����ˮ��Һ�������״̬�¶�������Ļ�������ʡ������Ȳ��ǵ����Ҳ���Ƿǵ���ʣ�
ǿ���������ˮ��Һ�л�����״̬������ȫ����ĵ���ʣ�����ǿ�ᡢǿ����ý���������ʹ��Σ�
��3����ˮϡ������ʱ����ٽ�����ĵ��룻�����кͷ�Ӧ�����ĵ������ӵ����ʵ����������������ӵ����ʵ������
��4���������Һ��pHΪa������Һ��pHΪb�����ݸ��¶��Լ������ϵ��ʽ���㣮
��� �⣺��1�������Ȼ���Ϊǿ����ʣ���ȫ����0.1mol•L-1������������Ũ��Ϊ0.1mol•L-1��������Ϊǿ�����0.2mol•L-1�����ᣬ������Ũ��Ϊ��0.4mol•L-1�����۴���Ϊ������ʣ����ֵ��룬0.1mol•L-1�Ĵ���������Ũ��С��0.1mol•L-1����һˮ�ϰ�Ϊ������ʣ����ֵ��룬0.1mol•L-1�İ�ˮ����������Ũ��С��0.1mol•L-1��������������Ϊǿ����ʣ���ȫ���룬0.1mol•L-1��NaOH��Һ������������Ũ��0.1mol•L-1������������Ϊǿ����ʣ���ȫ���룬0.1mol•L-1��Ba��OH��2��Һ������������Ũ��Ϊ0.2mol•L-1��
������Ũ��Խ��pHԽС������������Ũ��Խ��pHԽ������pH��С�����˳�ڢ٢ۢܢݢޣ�
�ʴ�Ϊ���ڢ٢ۢܢݢޣ�
��2����NaCl���Σ�����״̬��������ˮ�ܹ���ȫ���룬����ǿ����ʣ�����BaSO4��������״̬�ܹ���ȫ���룬����ǿ����ʣ�
��H2SO4��ǿ�ᣬ����ˮ�ܹ���ȫ���룬����ǿ����ʣ���KOH��ǿ�����ˮ��������״̬����ȫ��������ǿ����ʣ�
��ʯī�ǵ��ʼȲ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ�����H2CO3������ˮ��Һ���ܲ��ֵ��룬����������ʣ�
��CH3COOH��������ˮ��Һ���ܲ��ֵ��룬����������ʣ���NH3•H2O �����ˮ��Һ���ܲ��ֵ��룬����������ʣ� ��������ˮ��Һ������״̬�������磬���ڷǵ���ʣ�����ƾ���ˮ��Һ������״̬�������磬���ڷǵ���ʣ�������11��SO2���������ܵ��룬���ڷǵ���ʣ���12��Cu ���ʣ��Ȳ��ǵ����Ҳ���Ƿǵ���ʣ�
��13��Cl2 �ǵ��ʼȲ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ���14��CO2���������ܵ��룬���ڷǵ���ʣ�
����ǿ����ʵ��У��٢ڢۢܣ�������������ʵ��У��ޢߢࣻ�������ڷǵ���ʵ��Т�⣨11����14����
�ʴ�Ϊ���٢ڢۢܣ��ޢߢࣻ��⣨11����14����
��3��ijһԪ������Һ��A�����Ԫǿ�ᣨB����pH��ȣ��������д�Ϊ���룬��ˮϡ��ʱ���ܹ��ٽ������������룬���Լ�ˮϡ�ͺ�������Ũ�ȴ��ڶ�Ԫǿ����������Ũ�ȣ�����pHA��pHB��
����ϡ����Һ������������ӵ����ʵ���Ũ�ȴ���ǿ�ᣬ���Ե�������е������ӵ����ʵ����࣬�к͵�Ũ�ȵ������NaOH��Һ���õ�����٣�
�ʴ�Ϊ����������
��4����ǿ����Һ��pHΪa�����ΪV��pH=-lgc��H+������Һ��������Ũ��Ϊ��10-amol/L������Һ��pHΪb�����Ϊ10V����Һ�����������ӵ�Ũ��Ϊ��c��OH-��=10-��14-b��mol/L����Ϻ���Һ�����ԣ���������Һ�������ӵ����ʵ����������������ӵ����ʵ�������10-amol/L��VL=10-��14-b��mol/L��10VL��
��ã�-a=b-13��a+b=13����pH��+pH��=13��
�ʴ�Ϊ��pH��+pH��=13��
���� ���⿼��������Ũ�ȴ�С�ıȽϣ�����ʡ�ǿ����ʡ��ǵ���ʵ��жϣ���Һ�������pHֵ����ؼ��㣬��Ŀ�ۺ���ǿ��������ݶ࣬�Ѷ��еȣ�����ʱע����յ����ǿ�����ж����ݣ�ע��������ʵ�����ص㣮

| A�� | C3H6��C4H8 | B�� | C3H6��C4H6O | C�� | C2H6��C3H6O2 | D�� | CH4O��C3H4O2 |
| A�� | ����ë | B�� | ճ����ά | C�� | ë���� | D�� | �� |
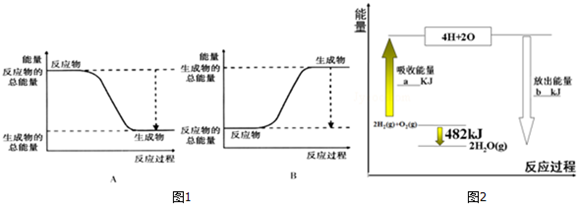
��1����֪�÷�ӦΪ���ȷ�Ӧ��ͼ1����ȷ��ʾ�÷�Ӧ�������仯����A��
��2����֪����1mol��̬������ij�ֹ��ۼ���Ҫ���յ����������Ǹù��ۼ��ļ��ܣ��±���ijЩ���ۼ��ļ��ܣ�
| ���ۼ� | H-H | O=O | H-O |
| ����/kJ•mol-1 | 436 | 498 | X |
��ͼ2�У�a=1370��
�ڱ����У�X=463��
����д���÷�Ӧ���Ȼ�ѧ����ʽ��2H2��g��+O2��g��=2H2O ��g����H=-482KJ/mol��
��3������ȼ�ϵ�ص��ܷ�Ӧ����ʽΪ2H2+O2�T2H2O�����У������ڸ��������������������������Ӧ�����������ԭ��������·��ÿת��0.2mol���ӣ���״��������������������3.36 L��
| A�� | ��ʯ��������炙����ĥ NH4++OH-�TNH3��+H2O | |
| B�� | ��KOH��Һ��ͨ��������SO2 ���� SO2+OH-�THSO3- | |
| C�� | ͭƬͶ��ϡ������ 3Cu+8H++2 NO3-�T3Cu2++2NO��+4H2O | |
| D�� | ����ͨ��ˮ�� Cl2+H2O�T2 H++Cl-+ClO- |
| A�� | K+��Cu2+��Cl-��SO42- | B�� | NO3-��Mg2+��S2-��SO42- | ||
| C�� | HCO3-��I-��Ca2+��Cl- | D�� | K+��Ca2+��CO32-��Cl- |
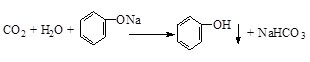 ��
��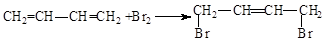 ��
�� �л���A��һ�ֺ������������ʽΪC6H9O2Br����֪�����µ�ת����ϵ��
�л���A��һ�ֺ������������ʽΪC6H9O2Br����֪�����µ�ת����ϵ��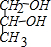 ��HOCH2CH2CH2OH��
��HOCH2CH2CH2OH��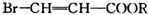 ��R��ʾ����������2�֣�
��R��ʾ����������2�֣�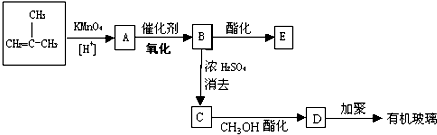
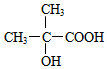 C
C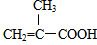 E
E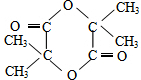
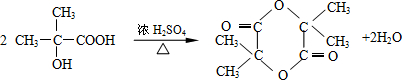 ��
��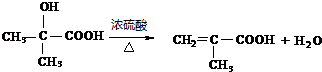 ��
��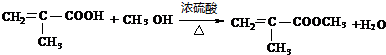 ��
��