��Ŀ����
��6�֣�A��B��C��D��4��Ԫ�أ�AԪ��������������������������ԭ����������ȣ�B��ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ������2����C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��
(1)B�����ڱ��е�λ�õ� ���ڣ��� �壻
(2)A��B�γɵĻ�����ĵ���ʽ ��
(3)C��Ԫ������___ _____��C�����������Ļ�ѧʽ ��
(4) D������������Ӧ��ˮ����Ļ�ѧʽ ��
(1)B�����ڱ��е�λ�õ� ���ڣ��� �壻
(2)A��B�γɵĻ�����ĵ���ʽ ��
(3)C��Ԫ������___ _____��C�����������Ļ�ѧʽ ��
(4) D������������Ӧ��ˮ����Ļ�ѧʽ ��
��6�֣�(ÿ��1��)
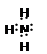 (1)�� �� ���ڣ��� VA �壻
(1)�� �� ���ڣ��� VA �壻
(2) �� (3) �� SO3��(4) KOH
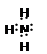 (1)�� �� ���ڣ��� VA �壻
(1)�� �� ���ڣ��� VA �壻(2) �� (3) �� SO3��(4) KOH
A���⣬HBO3���������+5�ۣ�ԭ�Ӱ뾶����������������С�ģ�B�ǵ���CԪ��ԭ�ӵ������������ȴ������2����C������D2C��DΪ��1�ۣ���K��A��B��C��D 4��Ԫ�طֱ�ΪH��N��S��K��
(1)N�ڵ������ڣ��� VA�壻
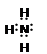 (2)NH3�ĵ���ʽΪ �� (3) �� SO3��(4) KOH
(2)NH3�ĵ���ʽΪ �� (3) �� SO3��(4) KOH
��ϰ��ϵ�д�
�����Ŀ