��Ŀ����
10��ij��ȤС���ͭ��Ũ���ᷴӦ�����ĺ�ɫ���������ܺ���CuO��CuS��Cu2S������CuS�� Cu2S������ϡ���ᡢϡ���ᣩ����̽����ʵ�鲽�����£�������ͭ˿����Ũ���ᣬ���ȣ�
������������ɫ����������ʱ�����ͭ˿��ֹͣ���ȣ�
����ȴ�ӷ�Ӧ��Ļ�����з������ɫ������ϴ�������ﱸ�ã�
�ش��������⣺
��1��������������Ļ�ѧʽΪSO2��
��2������ Cu2+��Һ�еμ�K4[Fe��CN��6]��Һ���ܲ������ɫ�������ֽ�������ɫ��������ϡ�����У�������Ժ��ٵμ�K4[Fe��CN��6]��Һ��δ�����ɫ�������ɴ����ý����Ǻ�ɫ��������CuO��
��3��Ϊ֤����ɫ��������ͭ�������������ʵ�飺
| װ�� | ���� | ���ۼ����� |
 | ��A�Թ��к�ɫ�������ܽ� ��A�Թ��Ϸ����ֺ���ɫ���� ��B�Թ��г��ְ�ɫ���� | a�������˵����ɫ�������� ��ԭ�ԣ� b���Թ�B�в�����ɫ�������ܷ�Ӧ�����ӷ���ʽΪ NO2+SO2+Ba2++H2O=BaSO4��+NO+2H+ |
��5��Ϊ�ⶨ��ɫ������Cu2S �İٷֺ�����ȡ0.2g ��������ú�ɫ��������������Һ���� 40.0mL 0.075mol/L KMnO4��Һ������������Ӧ���£�
8MnO4-+5Cu2S+44H+�T10Cu2++5SO2��+8Mn2++22H2O
6MnO4-+5CuS+28H+�T5Cu2++5SO2��+6Mn2++14H2O
��Ӧ�������Һ���Ͼ�SO2�������ĸ��������Һǡ����35.0mL 0.1mol/L ��NH4��2Fe��SO4��2 ��Һ��Ӧ��ȫ����������Cu2S ����������Ϊ40%��
���� ��1������ͭ��Ũ���ᷴӦ���ɶ�������������н��
��2������������Ϣ�м���ͭ���ӵķ����Ԣڽ��з�����Ȼ��ó���ȷ���ۣ�
��3��a������ɫ����Ϊ����������˵��ϡ���ᱻ��ԭ����һ����������ɫ������л�ԭ�ԣ�
b�����ݷ�Ӧ����ۿ�֪��ɫ������ϡ���ᷴӦ�����˶�������֤����ɫ�����к�����Ԫ�أ�������������������Ļ�������ܹ����Ȼ�����Ӧ�������ᱵ�������ݴ�д����Ӧ�����ӷ���ʽ��
��4��CuS����Һ�д��ڳ����ܽ�ƽ�⣬����ƽ���ƶ�������
��5�����ݵζ�ʵ�����ݼ���ʣ�����������ʵ������õ�������ͭ����ͭ��Ӧ�ĸ���������ʵ��������ݷ�Ӧ�����ӷ���ʽ��ʽ����õ�����
��� �⣺��1��Cu��Ũ���ᷴӦ��������ͭ�����������ˮ����ӦΪCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+2SO2��+2H2O������������������SO2��
�ʴ�Ϊ��SO2��
��2������Һ�еμ�K4[Fe��CN��6]��Һ�����������ɫ������֤����Cu2+�����ݢڽ���ɫ��������ϡ�����У�һ��ʱ��μ�K4[Fe��CN��6]��Һ��δ�����ɫ������֪����ɫ������һ������CuO��
�ʴ�Ϊ����ɫ�����в�����CuO��
��3��a��A�Թ����Ϸ����ֺ���ɫ���壬˵����Ӧ����һ���������ɣ�֤���˺�ɫ������л�ԭ�ԣ��ڷ�Ӧ�б�������
�ʴ�Ϊ����ԭ�ԣ�
b�����ݷ�Ӧ�����B�Թ��г��ְ�ɫ������֪����ɫ����Ϊ���ᱵ��˵����ɫ�����к�����Ԫ�أ�������Ӧ�����ӷ���ʽΪ��NO2+SO2+Ba2++H2O�TBaSO4��+NO��+2H+��
�ʴ�Ϊ��B�Թ��г��ְ�ɫ������NO2+SO2+Ba2++H2O�TBaSO4��+NO��+2H+��
��4��CuS������ˮ����ˮ��Һ�л��к�������Cu�ܽ⣬��Һ�д��ڳ����ܽ�ƽ�⣬CuS��s��?Cu2+��aq��+S2-��aq�����ȵ�Ũ���ὫS2-������ʹS2-Ũ�ȼ�С���ٽ�����ƽ���������ƶ���ʹCuS�ܽ⣻
�ʴ�Ϊ��CuS�����ܽ�ƽ��CuS��s��?Cu2+��aq��+S2-��aq�����ȵ�Ũ���ὫS2-������ʹS2-Ũ�ȼ�С���ٽ�����ƽ���������ƶ���ʹCuS�ܽ⣻
��5�������ķ�ӦΪ��
8MnO4-+5Cu2S+44H+�T10Cu2++5SO2��+8Mn2++22H2O
6MnO4-+5CuS+28H+�T5Cu2++5SO2��+6Mn2++14H2O
MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O
��Cu2S��CuS�����ʵ����ֱ�Ϊx��y��
��Cu2S��CuS��Ӧ��ʣ��KMnO4�����ʵ�����0.035L��0.1mol/L��$\frac{1}{5}$=0.0007mol��
160x+96y=0.2
$\frac{8x}{5}$+$\frac{6y}{5}$=0.04��0.075-0.0007
���x=0.0005mol��
Cu2S������������$\frac{0.0005mol��160g/mol}{0.2g}$��100%=40%��
�ʴ�Ϊ��40%��
���� ���⿼����Ũ����Ļ�ѧ���ʡ�����ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ������漰�������Դ�֪ʶ��϶࣬����������Ϣ�ǽ���ؼ�������������ѧ���ķ���������������

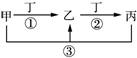 �ס��ҡ�����������ѧ���������ʣ����мס��ҡ���������ͬһ��Ԫ�أ���һ�������µ�ת����ϵ��ͼ������˵����ȷ���ǣ�������
�ס��ҡ�����������ѧ���������ʣ����мס��ҡ���������ͬһ��Ԫ�أ���һ�������µ�ת����ϵ��ͼ������˵����ȷ���ǣ�������| A�� | ����Ϊ���������;���Ľ������ʣ��ҵ���Һһ��ΪFeCl3 | |
| B�� | ��ͨ������¼ס��ҡ��������������壬���ҺͶ�Ϊ��������Ҫ�ɷ֣���Ӧ�ٵĻ�ѧ����ʽΪ4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O | |
| C�� | ���ס��ҡ�������Һ�Լ��ԣ�������ܿ�����Ϊҽ��������θ�����֢��ҩ�� | |
| D�� | ����Ϊ�������Ϊ�ȼҵ����Ҫ��Ʒ�����һ��Ϊ��Al3+���� |
| Ԫ�ش��� | L | M | Q | R | T |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.089 | 0.104 | 0.066 |
| ��Ҫ���ϼ� | +2 | +3 | +2 | +6��-2 | -2 |
| A�� | �⻯��ķе�ΪH2T��H2R | B�� | ������ϡ���ᷴӦ�Ŀ���ΪL��Q | ||
| C�� | M��T�γɵĻ����������ӻ����� | D�� | L2+��R2-�ĺ����������� |
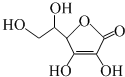
| A�� | ά����C�����Ĺ��������ǻ����Ȼ���̼̼˫�� | |
| B�� | ά����C�ܺ���ˮ�������ظ������Һ��Ӧ | |
| C�� | ά����C�ķ���ʽΪC6H6O6 | |
| D�� | ά����C�ܷ����ӳɷ�Ӧ��������Ӧ�����ܷ���ȡ����Ӧ |
| A�� | 3.0 L 0.1 mol•L-1 NaOH��Һ�л���ͨ��CO2����Һ����8.8 gʱ����Һ�У�c��Na+����c��CO32-����c��HCO3-����c��OH-����c��H+�� | |
| B�� | �����£���CH3COONa��Һ��ϡ����������ҺpH=7�� c��Na+����c��CH3COO-����c��Cl-��=c��CH3COOH����c��H+��=c��OH-�� | |
| C�� | �����£�pH=3�Ĵ�����Һ��pH=13������������Һ�������Ϻ����ǻ�Ϻ���Һ����ı仯����ǡ����ȫ��Ӧ����ԭ������Һ����ĵ����Ϊ1% | |
| D�� | ���ʵ���Ũ��֮��Ϊ1��2��NaClO��NaHCO3�����Һ�У�c��HClO��+c��ClO-��=2c��HCO3-��+2c��H2CO3��+2c��CO32-�� |
��1����PM2.5����������ˮ�����Ƴɴ�����������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
��2��NOx ����β������Ҫ��Ⱦ��֮һ����������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�
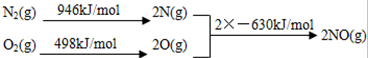
��N2��O2��Ӧ����NO���Ȼ�ѧ��Ӧ����ʽΪN2��g��+O2��g��=2NO��g����H=+184kJ•mol-1
��3����ѭ�����ղ���������SO2���ͻ�����Ⱦ��ͬʱ�����Ƶ������������������£�
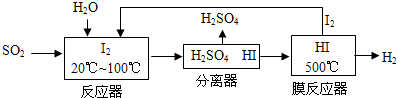
�������ӷ���ʽ��ʾ��Ӧ���з����ķ�Ӧ��SO2+I2+2H2O=SO42-+2I-+4H+
�ڽ����ɵ������������ֱ�ͨ����������Ե缫��KOH��Һ��Ϊ�������Һ�������ĵ缫��ӦʽH2-2e-+2OH-=2H2O
��4��Ϊ�˸��ƻ������й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%��50%��
����Ч����̼�����ֶ�֮һ�ǽ��ܣ��������ⷽ������ܵ���C������ţ���
A�����ˮ���⣺2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2��B������ʹˮ�ֽ����⣺2H2O��g��$\frac{\underline{\;����\;}}{\;}$2H2+O2
C��̫������ֽ�ˮ���⣺2H2O$\frac{\underline{\;\;\;TiO_{2}\;\;\;}}{̫����}$2H2��+O2��
D����Ȼ�����⣺CH4+H2O��g��$\frac{\underline{\;����\;}}{\;}$CO+3H2
��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1L���ܱ������У�����1mol CO2��3mol H2��һ�������·�Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H=-49.0kJ•mol-1�����CO2��CH3OH��g��Ũ����ʱ��仯��ͼ��ʾ����3min��9min��v��H2��=0.125mol•L-1•min-1��
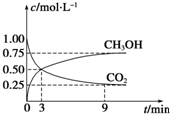
����˵��������Ӧ�ﵽƽ��״̬����D�����ţ���
A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1
����ͼ�н���㣩
B�����������ܶȲ���ʱ��ı仯���仯
C����λʱ��������3mol H2��ͬʱ����1mol H2O
D��CO2����������ڻ�������б��ֲ���
�ܹ�ҵ�ϣ�CH3OHҲ����CO��H2�ϳɣ��ο��ϳɷ�ӦCO��g��+2H2��g��?CH3OH��g����ƽ�ⳣ��������˵����ȷ����AC��
| �¶�/�� | 0 | 100 | 200 | 300 | 400 |
| ƽ�ⳣ�� | 667 | 13 | 1.9��10-2 | 2.4��10-4 | 1��10-5 |
B���÷�Ӧ�ڵ����²����Է����У������¿��Է�����
C����T��ʱ��1L�ܱ������У�Ͷ��0.1mol CO��0.2mol H2���ﵽƽ��ʱ��COת����Ϊ50%�����ʱ��ƽ�ⳣ��Ϊ100
D����ҵ�ϲ����Ըߵ�ѹǿ��5MPa����250�棬����Ϊ�������£�ԭ����ת������ߣ�
| A�� | ����ϩʳƷ��װ����ʳ�ﱣ��Ĥ�������ĸ߷��Ӳ��� | |
| B�� | ������ɱ�����в�������Ϊ���ɲ����ĵ��������ȱ��� | |
| C�� | ̫���ܵ�ذ��еĹ��ǹ赥�ʣ����ά����Ҫ�ɷ��Ƕ������� | |
| D�� | ����[KAl��SO4��2•12H2O]����������ˮ�ľ�����ɱ������ |
| ���� | ���� | �Լ� | �ᴿ���� | |
| A | �� | ���� | ��ˮ | ���� |
| B | ������̼ | �������� | ����̼������Һ | ϴ�� |
| C | �������� | ���� | ϡ����������Һ | ��������÷�Һ |
| D | ������ | ���� | �����������Һ | ���������ˡ�ϴ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |