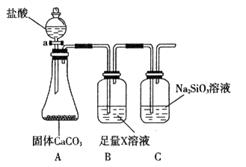
��Ŀ����
��ÿ��2�֣���16�֣�������Ԫ��X��Y��Z��W��ԭ���������������³�ѹ�£�ֻ��W�ĵ���Ϊ���塣���ǵ�����������Ӧ��ˮ��������Ϊ�ס��ҡ����������ס��ҡ�������ѧ��ѧ�еij������ʣ�����ֻ����������ˮ�����ܺͼס�����Ӧ�õ�������Һ������������Ϣ��д���пհף�
�Ż���W��ԭ�ӽṹʾ��ͼ_________________________________________��
�ƽ��Һͼס����ֱ�Ӧ��õ�����Һ��ϣ��۲쵽��������___________________
___________________ ������Һ���ʱ��������Ӧ�����ӷ���ʽΪ____________________________________________________��
��������ʵ��֤��Z��W�ǽ�����ǿ�����ǣ�ѡ����ţ�__________________________��
E.�⻯��ˮ��Һ�����ԣ�HW��H2Z
F.�ܽ��ԣ�������
����Y���ʺ���������õĽ������缫���õ������Ӳ������Һ�й���ԭ��أ���ԭ��ظ����ĵ缫��ӦʽΪ_______________________________________________ ��
�ɹ�ҵ����XWΪԭ�Ͽ��Խ��������������W2��������Ҫ��Ʒ��д����ҵ����XWΪԭ��������W2�Ļ�ѧ����ʽ_______________________________________________
_______________________________________________����Ҫ����80.0 kg�����ʣ�������ҪXW______________kg��ͬʱ�ɵ�W2_____________m3���������
�Ż���W��ԭ�ӽṹʾ��ͼ_________________________________________��
�ƽ��Һͼס����ֱ�Ӧ��õ�����Һ��ϣ��۲쵽��������___________________
___________________ ������Һ���ʱ��������Ӧ�����ӷ���ʽΪ____________________________________________________��
��������ʵ��֤��Z��W�ǽ�����ǿ�����ǣ�ѡ����ţ�__________________________��
| A�����ʵ��۵㣺Z��W2 |
| B�����ԣ������� |
| C������Һ�У�W2��H2Z��2HW��Z |
| D���ȶ��ԣ�HW��H2Z |
F.�ܽ��ԣ�������
����Y���ʺ���������õĽ������缫���õ������Ӳ������Һ�й���ԭ��أ���ԭ��ظ����ĵ缫��ӦʽΪ_______________________________________________ ��
�ɹ�ҵ����XWΪԭ�Ͽ��Խ��������������W2��������Ҫ��Ʒ��д����ҵ����XWΪԭ��������W2�Ļ�ѧ����ʽ_______________________________________________
_______________________________________________����Ҫ����80.0 kg�����ʣ�������ҪXW______________kg��ͬʱ�ɵ�W2_____________m3���������
�� ���Ʋ�����ɫ��״������ Al3+��3AlO2����6H2O��4Al(OH)3�� ��
���Ʋ�����ɫ��״������ Al3+��3AlO2����6H2O��4Al(OH)3�� ��
�� B. C. D. �� Al��3e����4OH����AlO2����2H2O��
�� 2NaCl��2H2O 2NaOH��Cl2����H2�� 117 22.4
2NaOH��Cl2����H2�� 117 22.4
 ���Ʋ�����ɫ��״������ Al3+��3AlO2����6H2O��4Al(OH)3�� ��
���Ʋ�����ɫ��״������ Al3+��3AlO2����6H2O��4Al(OH)3�� ���� B. C. D. �� Al��3e����4OH����AlO2����2H2O��
�� 2NaCl��2H2O
 2NaOH��Cl2����H2�� 117 22.4
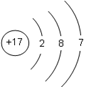
2NaOH��Cl2����H2�� 117 22.4 ����Ԫ�صĽṹ���й����ʿ�֪��X��Y��Z��W�ֱ���Na��Al��S��Cl��
��1����Ԫ�ص�ԭ������Ϊ17������ԭ�ӽṹʾ��ͼ��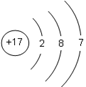 ��
��
��2���Һͼס����ֱ�Ӧ��õ�����Һƫ�����ƺ������������������������������������ʽΪAl3+��3AlO2����6H2O��4Al(OH)3����
��3���ǽ����ԵıȽϹ��ɣ�
1����Ԫ��ԭ�ӵ��������жϣ�һ������£�������Խǿ����Ӧ�ǽ�����Խǿ��
2���ɵ��ʺ�����ߺ�ˮ�ķ�Ӧ�̶��жϣ���ӦԽ���ң��ǽ�����Խǿ��
3���ɶ�Ӧ�⻯����ȶ����жϣ��⻯��Խ�ȶ����ǽ�����Խǿ��
4���ɺ��������ϵ����׳̶��жϣ�����Խ���ף��ǽ�����Խǿ��
5��������������Ӧˮ������������жϣ�����Խǿ���ǽ���Խǿ��������Ԫ��֮�⣩
6���ɶ�Ӧ�����ӵĻ�ԭ���жϣ���ԭ��Խǿ����Ӧ�ǽ�����Խ����
7�����û���Ӧ�жϣ�ǿ���������������û���Ӧ��˵��Ԫ�صķǽ�����ǿ������ǽ�������Ӧ�����������ǽ�����������ԭ�����û���Ӧ������Ϊ�ȽϷǽ�����ǿ�������ݡ�
ֵ��ע����ǣ���Ԫ��û������̬����û�з��ĺ����ᣬ��������������Ӧˮ�����������ǿ���Ǹ����ᣬ�����Ƿǽ����Ը����ȵķ�Ԫ�أ��ʹ���5ֻ�����ڷ�Ԫ��֮��ķǽ���Ԫ�ء�
8����Ԫ�������ɣ�ͬ����Ԫ�������ң���˵���������ӣ��ǽ�������ǿ��ͬ����Ԫ�����ϵ��£���˵���������ӣ��ǽ����Լ�����
�ݴ˿�֪ѡ��BCD��ȷ��
��4������������Ľ����������������Ļ�����ǿ�����ģ����ܺ��������Ʒ�Ӧ���������Ǹ���������������������ӦʽΪ Al��3e����4OH����AlO2����2H2O��
��5�����Ե缫���������Һ�ķ���ʽΪ2NaCl��2H2O 2NaOH��Cl2����H2�� 80.0kg����������80000g��40g/mol��2000mol��������Ҫ�Ȼ�����2000mol��������117kg��ͬʱ����������1000mol�����״���µ������22.4m3.
2NaOH��Cl2����H2�� 80.0kg����������80000g��40g/mol��2000mol��������Ҫ�Ȼ�����2000mol��������117kg��ͬʱ����������1000mol�����״���µ������22.4m3.
��1����Ԫ�ص�ԭ������Ϊ17������ԭ�ӽṹʾ��ͼ��
 ��
����2���Һͼס����ֱ�Ӧ��õ�����Һƫ�����ƺ������������������������������������ʽΪAl3+��3AlO2����6H2O��4Al(OH)3����
��3���ǽ����ԵıȽϹ��ɣ�
1����Ԫ��ԭ�ӵ��������жϣ�һ������£�������Խǿ����Ӧ�ǽ�����Խǿ��
2���ɵ��ʺ�����ߺ�ˮ�ķ�Ӧ�̶��жϣ���ӦԽ���ң��ǽ�����Խǿ��
3���ɶ�Ӧ�⻯����ȶ����жϣ��⻯��Խ�ȶ����ǽ�����Խǿ��
4���ɺ��������ϵ����׳̶��жϣ�����Խ���ף��ǽ�����Խǿ��
5��������������Ӧˮ������������жϣ�����Խǿ���ǽ���Խǿ��������Ԫ��֮�⣩
6���ɶ�Ӧ�����ӵĻ�ԭ���жϣ���ԭ��Խǿ����Ӧ�ǽ�����Խ����
7�����û���Ӧ�жϣ�ǿ���������������û���Ӧ��˵��Ԫ�صķǽ�����ǿ������ǽ�������Ӧ�����������ǽ�����������ԭ�����û���Ӧ������Ϊ�ȽϷǽ�����ǿ�������ݡ�
ֵ��ע����ǣ���Ԫ��û������̬����û�з��ĺ����ᣬ��������������Ӧˮ�����������ǿ���Ǹ����ᣬ�����Ƿǽ����Ը����ȵķ�Ԫ�أ��ʹ���5ֻ�����ڷ�Ԫ��֮��ķǽ���Ԫ�ء�
8����Ԫ�������ɣ�ͬ����Ԫ�������ң���˵���������ӣ��ǽ�������ǿ��ͬ����Ԫ�����ϵ��£���˵���������ӣ��ǽ����Լ�����
�ݴ˿�֪ѡ��BCD��ȷ��
��4������������Ľ����������������Ļ�����ǿ�����ģ����ܺ��������Ʒ�Ӧ���������Ǹ���������������������ӦʽΪ Al��3e����4OH����AlO2����2H2O��
��5�����Ե缫���������Һ�ķ���ʽΪ2NaCl��2H2O
 2NaOH��Cl2����H2�� 80.0kg����������80000g��40g/mol��2000mol��������Ҫ�Ȼ�����2000mol��������117kg��ͬʱ����������1000mol�����״���µ������22.4m3.
2NaOH��Cl2����H2�� 80.0kg����������80000g��40g/mol��2000mol��������Ҫ�Ȼ�����2000mol��������117kg��ͬʱ����������1000mol�����״���µ������22.4m3.
��ϰ��ϵ�д�
�����Ŀ


 �Ժ�ĸ���Ʒ������Ҫ�ɷ�Ϊ��ˮƽ�����Ե�
�Ժ�ĸ���Ʒ������Ҫ�ɷ�Ϊ��ˮƽ�����Ե�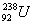 �������й�
�������й� ��
��