��Ŀ����
����Ŀ����.NOx��CO��SO2�ȴ�����Ⱦ����Ĵ�������������������о����ȵ����⡣
��1����֪��
a.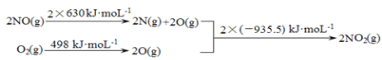
b.2NO��g����O2��g��![]() 2NO2��g�� ��H1
2NO2��g�� ��H1
2SO2��g����O2��g��![]() 2SO3��g�� ��H2=��196.6 kJ��mol-1
2SO3��g�� ��H2=��196.6 kJ��mol-1
����H1 =___kJ��mol-1��
��д��NO2������SO2���巴Ӧ����SO3�����NO������Ȼ�ѧ����ʽ____��
��2��ú̿ȼ�չ����в�����CO�ֻ���CaSO4������ѧ��Ӧ������������Ч�ʡ�������������ط�Ӧ���Ȼ�ѧ����ʽ���£�
��Ӧ��CaSO4��s����CO��g��![]() CaO��s����SO2��g����CO2��g�� ��H1��+218.4 kJ��mol-1
CaO��s����SO2��g����CO2��g�� ��H1��+218.4 kJ��mol-1
��Ӧ��CaSO4��s����4CO��g��![]() CaS��s����4CO2��g�� ��H2��-175.6 kJ��mol-1
CaS��s����4CO2��g�� ��H2��-175.6 kJ��mol-1
�����ϣ��ٷ�Ӧ��ͷ�Ӧ��ͬʱ�������ڷ�Ӧ������ʴ��ڷ�Ӧ������ʣ�
��ش��������⣺
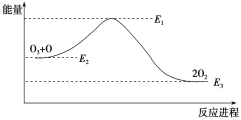
�����з�Ӧ���������仯ʾ��ͼ��ȷ����____��
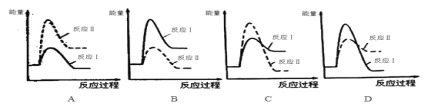
��.���淴ӦmA(g)��nB(g)![]() pC(g)��gD(g)��v��tͼ����ͼ����ʾ���������������䣬ֻ�ڷ�Ӧǰ������ʵĴ�������v��tͼ��������ʾ������˵����ȷ����___��
pC(g)��gD(g)��v��tͼ����ͼ����ʾ���������������䣬ֻ�ڷ�Ӧǰ������ʵĴ�������v��tͼ��������ʾ������˵����ȷ����___��
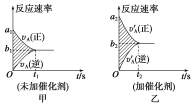
��a1��a2 ��a1��a2 ��b1��b2 ��b1��b2 ��t1��t2 ��t1��t2 ����ͼ����Ӱ���������� ��ͼ������Ӱ�����������
��.��0.5L���ܱ������У�һ�����ĵ����������������»�ѧ��Ӧ��N2(g)+3H2(g)![]() 2NH3(g) ��H��0���仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ�����
2NH3(g) ��H��0���仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ�����
T/�� | 200 | 300 | 400 |
K | K1 | K2 | 0.5 |
��ش�����������
���ԱȽ�K1��K2�Ĵ�С��K1____K2(�������=������)��
��400��ʱ����Ӧ2NH3(g)![]() N2(g)+3H2(g)��ƽ�ⳣ��KΪ____�������NH3��N2��H2��Ũ�ȷֱ�Ϊ3molL1��2molL1��1molL1ʱ����÷�Ӧ��v��(N2)___v��(N2)(�������=������)��
N2(g)+3H2(g)��ƽ�ⳣ��KΪ____�������NH3��N2��H2��Ũ�ȷֱ�Ϊ3molL1��2molL1��1molL1ʱ����÷�Ӧ��v��(N2)___v��(N2)(�������=������)��
���𰸡���113 NO2��g����SO2��g��![]() SO3��g����NO��g�� ��H=��41.8kJ��mol-1 C �ڢܢݢ� �� 2 ��
SO3��g����NO��g�� ��H=��41.8kJ��mol-1 C �ڢܢݢ� �� 2 ��
��������
��1���ٷ�Ӧ�ȵ��ڷ�Ӧ���м���֮�����������м���֮�͵IJ�ֵ������H1ͨ��ͼ����ʾ��֪Ӧ���ǣ�2��630+498��2��935.5��kJ��mol-1��-113 kJ��mol-1��
����֪��2NO��g����O2��g��![]() 2NO2��g�� ��H1��-113 kJ��mol-1
2NO2��g�� ��H1��-113 kJ��mol-1
2SO2��g����O2��g��![]() 2SO3��g�� ��H2=��196.6 kJ��mol-1
2SO3��g�� ��H2=��196.6 kJ��mol-1
���ݸ�˹���ɺ���ȥǰ���õ�NO2��g����SO2��g��![]() SO3��g����NO��g����H=��83.6kJ��mol-1�������Ȼ�ѧ����ʽΪNO2��g����SO2��g��
SO3��g����NO��g����H=��83.6kJ��mol-1�������Ȼ�ѧ����ʽΪNO2��g����SO2��g��![]() SO3��g����NO��g�� ��H=��41.8kJ��mol-1��
SO3��g����NO��g�� ��H=��41.8kJ��mol-1��
��2����.���ݻ��ԽС��ѧ��Ӧ����Խ�͵��ص㣬��Ϊ��Ӧ������ʴ��ڷ�Ӧ������ʣ�ͬʱ��Ӧ��Ϊ���ȷ�Ӧ����Ӧ��Ϊ���ȷ�Ӧ������ѡC��
��. ��Ϊ���˴�������ѧ��Ӧ���ʿ죬���Ԣ���ȷ�����˴�����ƽ��ʱ��ѧ��Ӧ����Ҳ�ӿ죬���Ԣ���ȷ����Ϊ���˴�������ѧ��Ӧ���ʼӿ죬ʱ���̣����Ԣ���ȷ���ߴ���ֻ�ı�����û�ı�ƽ�⣬����������ʱ��ij˻����䣬������Ӱ�����ȣ����Ԣ���ȷ��
��.�������¶Ⱥ�ƽ���������ƶ�����ѧƽ�ⳣ����С������K1��K2��
��400��ʱ����Ӧ2NH3(g)![]() N2(g)+3H2(g)��ƽ�ⳣ��KΪ����Ӧ�Ļ�ѧƽ�ⳣ���ĵ���������Ϊ
N2(g)+3H2(g)��ƽ�ⳣ��KΪ����Ӧ�Ļ�ѧƽ�ⳣ���ĵ���������Ϊ![]() =2�������ʱ������
=2�������ʱ������![]() ��2������ƽ���������ƶ�������v��(N2)��v��(N2)��
��2������ƽ���������ƶ�������v��(N2)��v��(N2)��

����Ŀ��һ���¶��£����Ϊ2 L���ܱ�������X��Y��Z��������ij�ʼ���ʵ�����ƽ�����ʵ������±���
���� | X | Y | Z |
��ʼ���ʵ���/mol | 0.2 | 0.2 | 0 |
ƽ�����ʵ���/mol | 0.1 | 0.05 | 0.1 |
����˵����ȷ���ǣ� ��
A.��Ӧ�ɱ�ʾΪ![]() ����ƽ�ⳣ��Ϊ8 000
����ƽ�ⳣ��Ϊ8 000
B.����ѹǿʹƽ��������Z�ķ����ƶ�����ƽ�ⳣ������
C.�������������ѹ����1 L����X�����������С��Ũ������
D.�������¶ȣ�Z��Ũ���������¶�����ʱ![]() ������
������![]() ���С
���С