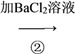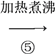��Ŀ����
����Ŀ����ˮ�к��и������࣬����70%Ϊ�Ȼ��ƣ���������Ȼ�þ������þ�ȡ�ij��ѧ��ȤС��Ϊ�˴Ӻ�ˮ�з�����Ȼ��ƣ����������ʵ�鷽����
��ˮ ��Һ
��Һ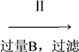 ��Һ
��Һ NaCl��Һ
NaCl��Һ![]() NaCl
NaCl
(1)��������Լ�AΪ________���������A��Ŀ����________��
(2)��������Լ�BΪ________���������B��Ŀ����________��
(3)��������Լ�CΪ_____���������C��______����Ŀ����________��
(4)�������ʵ�����Ϊ________���Ը���ͼʾ�ش�

�ٰ�˳��д��ͼ�б�����������ƣ�_________________________��
������a��������________����Ŀ����_________________________��
�۵�����b�г���________ʱ����ֹͣ���ȡ�
(5)�û�ѧ��ȤС���÷���õ����Ȼ�������100 mL 1 mol/L��NaCl��Һ�����ƹ���������������ƽ��ȡ���ε�����Ϊ______g�����ڶ��ݵIJ��������Ĺ���������______��
���𰸡� Ba(OH)2 ʹMg2����SO![]() ȫ��ת��Ϊ��������ȥ Na2CO3 ʹBa2��ȫ��ת��Ϊ̼�ᱵ��������ȥ ���� ��������� ��ȥ������CO
ȫ��ת��Ϊ��������ȥ Na2CO3 ʹBa2��ȫ��ת��Ϊ̼�ᱵ��������ȥ ���� ��������� ��ȥ������CO![]() ��OH�� �����ᾧ �������������ƾ��� ���� ��ֹ��ֲ����ȶ�����Һ�λ���ɽ� �������� 5.8(��5.9) 100 mL����ƿ
��OH�� �����ᾧ �������������ƾ��� ���� ��ֹ��ֲ����ȶ�����Һ�λ���ɽ� �������� 5.8(��5.9) 100 mL����ƿ
����������ˮ��70%Ϊ�Ȼ��ƣ���������Ȼ�þ������þ�ȣ�Ҫ�õ��ϴ�����NaCl��Ӧ�ó�ȥ��ˮ�е�Mg2+��SO42-��Mg2+��NaOH��Һ��ȥ������Mg(OH)2������SO42-��BaCl2��Һ��ȥ������BaSO4��������Ϊ������Ȼ�����Һ��������Na2CO3��ȥBaCl2��������ϡ�����ȥ������̼���ƣ����˺�õ�NaCl��Һ���������Ũ������ȴ�ᾧ�õ�NaCl���壻
���Ը�����ͼ��AΪNaOH��BaCl2��Һ��Ba(OH)2 ��Һ��BΪNa2CO3��Һ��CΪϡ���ᡣ
(1)������г�ȥþ���Ӻ���������ӣ��ֱ�����������þ�����ᱵ������������Ӧ�����ӷ���ʽ��Mg2++2OH-=Mg(OH)2����Ba2++SO42-=BaSO4�����������A��Ŀ����ʹMg2+��SO42-ȫ��ת��Ϊ��������ȥ���ʴ�Ϊ��NaOH��BaCl2��Һ��Ba(OH)2��Һ��ʹMg2+��SO42-ȫ��ת��Ϊ��������ȥ��
(2)��������Լ�Ŀ���dz�ȥ�������Ȼ�����BΪNa2CO3���ʴ�Ϊ��Na2CO3��ʹBa2+ȫ��ת��Ϊ̼�ᱵ������ȥ��
(3)��������Լ�Ŀ���dz�ȥ������̼���ƺ����������������Ҳ��������µ����ʣ�CΪϡ���ᣬ����������C��������������ʴ�Ϊ������������������ȥ������CO32-��OH-��
(4)�������ʵ�����Ϊ�������ʴ�Ϊ��������
��aΪ��������b��������c�Ǿƾ��ƣ��ʴ�Ϊ���������������ƾ��ƣ�
������a�Dz���������������ã���ֹ�ֲ��¶ȹ��߶�����Һ�壬�Ӷ��ײ�����ȫ�¹ʣ��ʴ�Ϊ�����裻��ֹ��Һ�ֲ��¶ȹ��߶�����Һ�壻
�۵�����b�г��ִ�������ʱ����ֹͣ���ȣ��������Ƚ���Һ���ɣ��ʴ�Ϊ���������壮
��100mL 1molL-1��NaCl��Һ���Ȼ��Ƶ����ʵ���Ϊ0.1L��1mol/L=0.1mol������Ϊ��0.1mol��58.5g/mol=5.85g��������ƽ��ȷ��Ϊ0.1g������Ҫ����5.9��ʵ������Ҫ��100mL 1molL-1��NaCl��Һ����ѡ��100mL����ƿ���ʴ�Ϊ��5.9��100mL����ƿ��