��Ŀ����
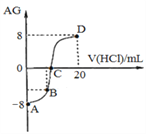
����Ŀ����һ���Ϊ1 L���ܱ������У�ͨ��һ������CO��H2O����850 �� �������·�Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ��
CO2(g)��H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ��

(1)0��4 min��ƽ����Ӧ����v(CO)��__________________mol��(L��min)��1��
(2)850 ��ʱ��ƽ�ⳣ��K��___________________��
(3)850 ��ʱ������������г���1.0 mol CO��3.0 mol H2O����CO��ƽ��ת����Ϊ___________��
(4)���жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������________(��ѡ�����)��
a��v��(H2)��v��(H2O) b��c(CO2)��c(CO)
c�������������ܶȲ��� d��1 mol H��H�����ѵ�ͬʱ����2 mol H��O��
���𰸡�0.03 1 75% ad
��������
��1��0-4min��֪����c��CO��=0.20mol/L-0.08mol/L=0.12mol/L����v��CO��=![]() =
=![]() 0.03mol��Lmin��-1���ʴ�Ϊ��0.03��
0.03mol��Lmin��-1���ʴ�Ϊ��0.03��
��2��ƽ�ⳣ��K= ![]() =
= ![]() =1
=1
��3����һ����̼��ת��Ũ��Ϊx��

850��ʱ����Ӧ��ƽ�ⳣ����1����(1.0-x)��(3.0-x)=x2�����x=0.75����CO��ƽ��ת����Ϊ0.751.0��100%=75%��
��4���÷�Ӧ���ڹ̶��ݻ����ܱ������н��еķ�Ӧ���ҷ�Ӧǰ��������������䣬�ܶȲ�����Ϊ�ﵽƽ��ı�־��c(CO2)=c(CO)��һ�ض���״̬����һ���ﵽƽ�⣬a��v��(H2)=v��(H2O)��d��1mol H-H�����ѵ�ͬʱ����2mol H-O������Ϊ�ﵽƽ��ı�־��