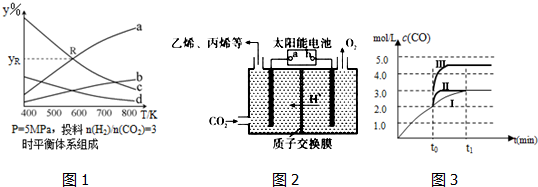
��Ŀ����
18�� �����£���20.00mL 0.100 0mol•L-1 NH3•H2O��Һ����εμ�0.1000mol•L-1 HCl��Һ����ҺpH�����HCl��Һ����ı仯��������ͼ��ʾ������˵����ȷ���ǣ�������
�����£���20.00mL 0.100 0mol•L-1 NH3•H2O��Һ����εμ�0.1000mol•L-1 HCl��Һ����ҺpH�����HCl��Һ����ı仯��������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ����Һ��c��Cl-����c��NH4+����c��OH-����c��H+�� | |
| B�� | ����Һ��c��NH4+��=c��Cl-����c��OH-��=c��H+�� | |
| C�� | �١��ڡ���������ʾ����Һ��ˮ�ĵ���̶Ȣڣ��ۣ��� | |
| D�� | �ζ������в����ܳ��֣�c��NH3•H2O����c��NH4+����c��OH-����c��Cl-����c��H+�� |
���� A���ٴ�����Ϊ��Ũ�ȵ��Ȼ�狀�һˮ�ϰ���笠����ӵĵ���̶ȴ���ˮ��̶ȣ���c��NH4+����c��Cl-����
B������Һ��pH=7����c��OH-��=c��H+������ϵ���غ��֪��c��NH4+��=c��Cl-����
C����Ϊ������Һ����Ϊ���ԣ��۴�����Ϊ�Ȼ�泥�笠�����ˮ��ٽ���ˮ�ĵ��룻
D����������Ȼ�������ʵ�������ʱ�����ܳ��ָ�����Ũ�ȹ�ϵ��
��� �⣺A���ٴ�����10mL���ᣬ��Ӧ������Ϊ��Ũ�ȵ��Ȼ�狀�һˮ�ϰ���笠����ӵĵ���̶ȴ���ˮ��̶ȣ���c��NH4+����c��Cl-������Һ������Ũ�ȴ�СΪ��c��NH4+����c��Cl-����c��OH-����c��H+������A����
B������Һ��pH=7������Һ��c��OH-��=c��H+������ϵ���غ��֪��c��NH4+��=c��Cl-����������Һ������Ũ�ȴ�СΪ��c��NH4+��=c��Cl-����c��OH-��=c��H+������B��ȷ��
C���ٴ���ˮ������������ˮ�ĵ��룬��Һ�е���������ˮ����ģ���Ϊ���ԣ�ˮ�����������Ϊ10-7mol/L���۴�����Ϊ�Ȼ�泥�笠�����ˮ��ٽ���ˮ�ĵ��룬���Ԣ۳�ˮ�ĵ���̶���ٴ�ˮ�ĵ���̶���С������ˮ�ĵ���̶ȴ�СΪ���ۣ��ڣ��٣���C����
D���ζ������У�������HCl�����ʵ�������ʱ������֣�c��NH3•H2O����c��NH4+����c��OH-����c��Cl-����c��H+������D����
��ѡB��
���� ���⿼��������ϵĶ����жϼ���ҺpH�ļ��㡢����Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ��Һ���������ҺpH�Ĺ�ϵ�����㷽��Ϊ���ؼ���ע�����յ���غ㡢�����غ㼰�ε�ˮ��ԭ�����ж�����Ũ�ȴ�С�е�Ӧ�ã�

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�| X | Y | ||
| W | Z | ||
| T |
| A�� | Xλ��Ԫ�����ڱ��еڶ����ڢ�A�� | |
| B�� | Y��Z�γ��⻯��ķе������Z | |
| C�� | ��X��Y��������Ԫ���γɵĻ�������ֻ�й��ۼ� | |
| D�� | Ԫ��T��X��ԭ���������25 |
| A�� | ���л��������NaOH��Һ��Ӧ | |
| B�� | ���л���ķ���ʽΪC13H19O3 | |
| C�� | ���л���ȿ��Է���������Ӧ���ܷ�����ԭ��Ӧ | |
| D�� | ���л���ͱ���������Ϊͬϵ�� |
| A�� | ��ϩʹ��ˮ�����Ը��������Һ��ɫ�����ڼӳɷ�Ӧ | |
| B�� | ���³�ѹ�»�����ϩ ��һ��������ˮ����ɫ���� ��һ��������ˮ����ɫ���� | |
| C�� | �������ظ������Һ����Ƽݣ������ķ�Ӧ�����Ҵ���������Ӧ | |
| D�� | ����������ϩ���Ҵ���ȫȼ�գ�����O2�����ʵ�����ͬ |
| ʵ�鲽��ͷ��� | ʵ������ |
| �ٰ�7ƿҺ��ֱ����α��A��B��C��D��E��F��G������ζ | ֻ��F��G����Һ��û����ζ |
| �ڸ�ȡ�������Թ��м�ˮϡ�� | ֻ��C��D��E����Һ�岻�ܽ������ˮ�ϲ� |
| �ֱ�ȡ����7��Һ�����Թ��м����Ƶ�Cu��OH��2������ | ֻ��Bʹ�����ܽ⣬F�в�����ɫ���� |
| ��ȡC��D��E�������Թ��У���ϡNaOH��Һ������ | ֻ��C���зֲ���������D���Թ����ŵ�������ζ |
A�Ҵ���B���ᡢC����D����������E��֬��F��������Һ��G������Һ��
��2��д����D�м���NaOH��Һ�����ȵĻ�ѧ����ʽ��CH3COOCH2CH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3CH2OH
��3����֪��ȩ����������Ӧ�Ļ�ѧ����ʽΪ��
CH3CHO+4[Ag��NH3��2]OH $\stackrel{��}{��}$ ��NH4��2CO3+4Ag��+6NH3��+2H2O��
��д�������Ƿ���������Ӧ�Ļ�ѧ����ʽΪCH2OH��CHOH��4CHO+2Ag��NH3��2OH$\stackrel{��}{��}$H2O+2Ag��+3NH3+CH2OH��CHOH��4COONH4��
| A�� | v��A��=0.15mol/��L•min�� | B�� | v��B��=0.3 mol/��L•min�� | ||
| C�� | v��C��=0.2 mol/��L•min�� | D�� | v��D��=0.1 mol/��L•min�� |
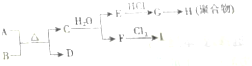
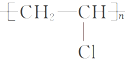 ��
��