ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ»θΒγΫβ÷ ΒΡΒγάκΤΫΚβΓΔ―ΈάύΒΡΥ°ΫβΤΫΚβΚΆΡ―»ήΈοΒΡ»ήΫβΤΫΚβΨυ τ”ΎΜ·―ßΤΫΚβΓΘ
(1)‘Ύ25Γφ ±Θ§ΫΪc molΓΛLΘ≠1ΒΡ¥ΉΥα»ή“Κ”κ0.02 molΓΛLΘ≠1 NaOH»ή“ΚΒ»ΧεΜΐΜλΚœΚσ»ή“ΚΗ’ΚΟ≥ ÷––‘Θ§”ΟΚ§cΒΡ¥ζ ΐ Ϋ±μ ΨCH3COOHΒΡΒγάκ≥Θ ΐKaΘΫ________ΓΘ
(2)25Γφ ±Θ§H2SO3![]() HSO3Θ≠ΘΪHΘΪΒΡΒγάκ≥Θ ΐKaΘΫ1ΓΝ10Θ≠2 molΓΛLΘ≠1Θ§‘ρΗΟΈ¬Ε»œ¬NaHSO3Υ°ΫβΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKbΘΫ________molΓΛLΘ≠1Θ§»τœρNaHSO3»ή“Κ÷–Φ”»κ…ΌΝΩΒΡI2Θ§‘ρ»ή“Κ÷–
HSO3Θ≠ΘΪHΘΪΒΡΒγάκ≥Θ ΐKaΘΫ1ΓΝ10Θ≠2 molΓΛLΘ≠1Θ§‘ρΗΟΈ¬Ε»œ¬NaHSO3Υ°ΫβΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKbΘΫ________molΓΛLΘ≠1Θ§»τœρNaHSO3»ή“Κ÷–Φ”»κ…ΌΝΩΒΡI2Θ§‘ρ»ή“Κ÷–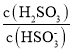 ΫΪ________(ΧνΓΑ‘ω¥σΓ±ΓΔΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±)ΓΘ
ΫΪ________(ΧνΓΑ‘ω¥σΓ±ΓΔΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±)ΓΘ
(3)“―÷ΣH2A‘ΎΥ°÷–¥φ‘Ύ“‘œ¬ΤΫΚβΘΚH2AΘΫHΘΪΘΪHAΘ≠Θ§HAΘ≠![]() HΘΪΘΪA2Θ≠ΓΘ≥ΘΈ¬œ¬H2AΒΡΗΤ―Έ(CaA)ΒΡ±ΞΚΆ»ή“Κ÷–¥φ‘Ύ“‘œ¬ΤΫΚβΘΚCaA(s)
HΘΪΘΪA2Θ≠ΓΘ≥ΘΈ¬œ¬H2AΒΡΗΤ―Έ(CaA)ΒΡ±ΞΚΆ»ή“Κ÷–¥φ‘Ύ“‘œ¬ΤΫΚβΘΚCaA(s)![]() Ca2ΘΪ(aq)ΘΪA2Θ≠(aq) ΓςH>0ΓΘ»τ“Σ ΙΗΟ»ή“Κ÷–Ca2ΘΪ≈®Ε»±δ–ΓΘ§Ω…≤…»ΓΒΡ¥κ ©”–________ΓΘ
Ca2ΘΪ(aq)ΘΪA2Θ≠(aq) ΓςH>0ΓΘ»τ“Σ ΙΗΟ»ή“Κ÷–Ca2ΘΪ≈®Ε»±δ–ΓΘ§Ω…≤…»ΓΒΡ¥κ ©”–________ΓΘ
A.…ΐΗΏΈ¬Ε» B.ΫΒΒΆΈ¬Ε» C.Φ”»κNH4ClΨßΧε D.Φ”»κNa2AΙΧΧε
ΓΨ¥πΑΗΓΩ![]() 1012 ‘ω¥σ BD
1012 ‘ω¥σ BD
ΓΨΫβΈωΓΩ
(1)‘Ύ25Γφ ±Θ§ΫΪc molΓΛLΘ≠1ΒΡ¥ΉΥα»ή“Κ”κ0.02 molΓΛLΘ≠1 NaOH»ή“ΚΒ»ΧεΜΐΜλΚœΚσ»ή“ΚΗ’ΚΟ≥ ÷––‘Θ§¥Υ ±c(OH-)ΘΫc(H+)=10-7mol/LΘ§c(CH3COO-)= c(Na+)=0.01 mol/LΘ§c(CH3COOH)=(![]() - 0.01)mol/LΘ§¥ζ»κCH3COOHΒΡΒγάκ≥Θ ΐ±μ¥ο ΫΘ§Φ¥Ω…«σ≥ωKaΓΘ
- 0.01)mol/LΘ§¥ζ»κCH3COOHΒΡΒγάκ≥Θ ΐ±μ¥ο ΫΘ§Φ¥Ω…«σ≥ωKaΓΘ
(2)25Γφ ±Θ§H2SO3![]() HSO3Θ≠ΘΪHΘΪΒΡΒγάκ≥Θ ΐKaΘΫ1ΓΝ10Θ≠2 molΓΛLΘ≠1Θ§‘ρΗΟΈ¬Ε»œ¬NaHSO3Υ°ΫβΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKbΘΫ
HSO3Θ≠ΘΪHΘΪΒΡΒγάκ≥Θ ΐKaΘΫ1ΓΝ10Θ≠2 molΓΛLΘ≠1Θ§‘ρΗΟΈ¬Ε»œ¬NaHSO3Υ°ΫβΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKbΘΫ![]() ΘΜ»τœρNaHSO3»ή“Κ÷–Φ”»κ…ΌΝΩΒΡI2Θ§ΖΔ…ζΖ¥”Π4HSO3-+I2+H2O=SO42-+2I-+3H2SO3Θ§”…¥ΥΩ…ΒΟ≥ω»ή“Κ÷–
ΘΜ»τœρNaHSO3»ή“Κ÷–Φ”»κ…ΌΝΩΒΡI2Θ§ΖΔ…ζΖ¥”Π4HSO3-+I2+H2O=SO42-+2I-+3H2SO3Θ§”…¥ΥΩ…ΒΟ≥ω»ή“Κ÷– ΒΡ±δΜ·ΓΘ
ΒΡ±δΜ·ΓΘ
(3)≥ΘΈ¬œ¬H2AΒΡΗΤ―Έ(CaA)ΒΡ±ΞΚΆ»ή“Κ÷–¥φ‘Ύ“‘œ¬ΤΫΚβΘΚCaA(s)![]() Ca2ΘΪ(aq)ΘΪA2Θ≠(aq) ΓςH>0ΓΘ»τ“Σ ΙΗΟ»ή“Κ÷–Ca2ΘΪ≈®Ε»±δ–ΓΘ§ΫαΚœΧΊΒψΘ§Ω…“‘œϊΚΡCa2+ ΙΤΫΚβ’ΐœρ“ΤΕ·Μρ ΙΤΫΚβΡφœρ“ΤΕ·ΓΘ
Ca2ΘΪ(aq)ΘΪA2Θ≠(aq) ΓςH>0ΓΘ»τ“Σ ΙΗΟ»ή“Κ÷–Ca2ΘΪ≈®Ε»±δ–ΓΘ§ΫαΚœΧΊΒψΘ§Ω…“‘œϊΚΡCa2+ ΙΤΫΚβ’ΐœρ“ΤΕ·Μρ ΙΤΫΚβΡφœρ“ΤΕ·ΓΘ
(1) ‘Ύ25Γφ ±Θ§ΫΪc molΓΛLΘ≠1ΒΡ¥ΉΥα»ή“Κ”κ0.02 molΓΛLΘ≠1 NaOH»ή“ΚΒ»ΧεΜΐΜλΚœΚσ»ή“ΚΗ’ΚΟ≥ ÷––‘Θ§¥Υ ±c(OH-)ΘΫc(H+)=10-7mol/LΘ§ΫαΚœΒγΚ… ΊΚψΘ§c(CH3COO-)= c(Na+)=0.01 mol/LΘ§ΗυΨίΈοΝœ ΊΚψΘ§c(CH3COOH)=(![]() - 0.01)mol/LΘ§Ka=
- 0.01)mol/LȧKa=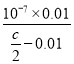 =
=![]() ΓΘ¥πΑΗΈΣΘΚ
ΓΘ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
(2)25Γφ ±Θ§H2SO3![]() HSO3Θ≠ΘΪHΘΪΒΡΒγάκ≥Θ ΐKaΘΫ1ΓΝ10Θ≠2 molΓΛLΘ≠1Θ§‘ρΗΟΈ¬Ε»œ¬NaHSO3Υ°ΫβΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKbΘΫ
HSO3Θ≠ΘΪHΘΪΒΡΒγάκ≥Θ ΐKaΘΫ1ΓΝ10Θ≠2 molΓΛLΘ≠1Θ§‘ρΗΟΈ¬Ε»œ¬NaHSO3Υ°ΫβΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKbΘΫ![]() =
=![]() molΓΛLΘ≠1=1012 molΓΛLΘ≠1ΘΜ»τœρNaHSO3»ή“Κ÷–Φ”»κ…ΌΝΩΒΡI2Θ§ΖΔ…ζΖ¥”Π4HSO3-+I2+H2O=SO42-+2I-+3H2SO3Θ§c(HSO3--)Φθ–ΓΘ§c(H2SO3)‘ω¥σΘ§
molΓΛLΘ≠1=1012 molΓΛLΘ≠1ΘΜ»τœρNaHSO3»ή“Κ÷–Φ”»κ…ΌΝΩΒΡI2Θ§ΖΔ…ζΖ¥”Π4HSO3-+I2+H2O=SO42-+2I-+3H2SO3Θ§c(HSO3--)Φθ–ΓΘ§c(H2SO3)‘ω¥σΘ§ ‘ω¥σΓΘ¥πΑΗΈΣΘΚ1012ΘΜ‘ω¥σΘΜ
‘ω¥σΓΘ¥πΑΗΈΣΘΚ1012ΘΜ‘ω¥σΘΜ
(3)A.…ΐΗΏΈ¬Ε»Θ§ΤΫΚβCaA(s)![]() Ca2ΘΪ(aq)ΘΪA2Θ≠(aq)’ΐœρ“ΤΕ·Θ§Ca2ΘΪ≈®Ε»±δ¥σΘ§A≤ΜΚœΧβ“βΘΜ
Ca2ΘΪ(aq)ΘΪA2Θ≠(aq)’ΐœρ“ΤΕ·Θ§Ca2ΘΪ≈®Ε»±δ¥σΘ§A≤ΜΚœΧβ“βΘΜ
B.ΫΒΒΆΈ¬Ε»Θ§ΤΫΚβCaA(s)![]() Ca2ΘΪ(aq)ΘΪA2Θ≠(aq)Ρφœρ“ΤΕ·Θ§Ca2ΘΪ≈®Ε»±δ–ΓΘ§BΖϊΚœΧβ“βΘΜ
Ca2ΘΪ(aq)ΘΪA2Θ≠(aq)Ρφœρ“ΤΕ·Θ§Ca2ΘΪ≈®Ε»±δ–ΓΘ§BΖϊΚœΧβ“βΘΜ
C.Φ”»κNH4ClΨßΧεΘ§NH4+Υ°Ϋβ…ζ≥…ΒΡH+”κA2Θ≠Ής”Ο…ζ≥…HA-Θ§ΤΫΚβ’ΐœρ“ΤΕ·Θ§Ca2ΘΪ≈®Ε»±δ¥σΘ§C≤ΜΚœΧβ“βΘΜ
D.Φ”»κNa2AΙΧΧεΘ§‘ω¥σ»ή“Κ÷–ΒΡc(A2-)Θ§ΤΫΚβΡφœρ“ΤΕ·Θ§Ca2ΘΪ≈®Ε»±δ–ΓΘ§DΖϊΚœΧβ“βΘΜ
¥πΑΗΈΣΘΚBDΓΘ

 –¬ΥΦΈ§ΦΌΤΎΉς“ΒΚ°ΦΌΦΣΝ÷¥σ―ß≥ωΑφ…γœΒΝ–¥πΑΗ
–¬ΥΦΈ§ΦΌΤΎΉς“ΒΚ°ΦΌΦΣΝ÷¥σ―ß≥ωΑφ…γœΒΝ–¥πΑΗ