��Ŀ����
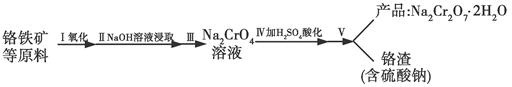
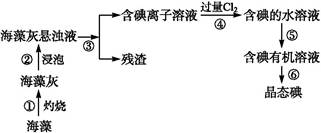
�Ժ���Al2O3��Fe2O3�����ʵĸ�����[��Ҫ�ɷ�ΪFe��CrO2��2]Ϊ��Ҫԭ�������ظ����ƾ��壨 Na2Cr2O7��2H2O������Ҫ�����������£�
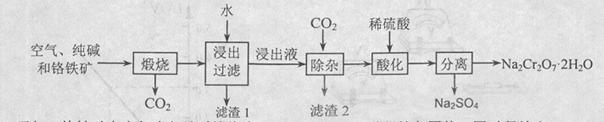
��֪���������ڿ������봿����������Na2CrO4��һ�ֺ���ɫ���壬ͬʱ�ͷų�CO2���壬ͬʱA12O3+Na2CO3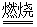 2NaAlO2+CO2������ش�
2NaAlO2+CO2������ش�
��1���ڸ�����Fe��CrO2��2�У�Cr�Ļ��ϼ�Ϊ____��
��2������1�ijɷ�Ϊ________������2�ijɷ�Ϊ____��
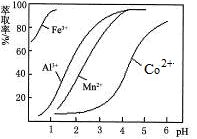
��3��������l����ϡ�����ܽ�����ҺW���������Һ�н������ӵķ����� ��
��4���������е�Al2O3�����ڹ�ҵ�Ͽ�����ұ�������û�ѧ����ʽΪ
��5�������йع��ұ�����CrO42���ķ�ˮҪ����ѧ������ʹ��Ũ�Ƚ���5��0��10��7 mo1/L���²����ŷš���CrO42���ķ�ˮ����ͨ�����������ַ�����
�ٳ���������������Ա�������BaCrO4���� ���ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С�� mol/L��������ˮ�������ܴﵽ�����ŷű���
���ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С�� mol/L��������ˮ�������ܴﵽ�����ŷű���
�ڻ�ԭ����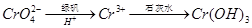 ��д������������ CrO42�����̷�����Һ�з�Ӧ�����ӷ���ʽ ��
��д������������ CrO42�����̷�����Һ�з�Ӧ�����ӷ���ʽ ��
��6��ij��Ч��ˮ������K2FeO4�õ��ģ���ҵ������ҺW��������غ���������Ϊԭ���Ʊ�K2FeO4���÷�Ӧ�Ļ�ѧ����ʽ�� ��

��֪���������ڿ������봿����������Na2CrO4��һ�ֺ���ɫ���壬ͬʱ�ͷų�CO2���壬ͬʱA12O3+Na2CO3
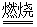 2NaAlO2+CO2������ش�
2NaAlO2+CO2������ش���1���ڸ�����Fe��CrO2��2�У�Cr�Ļ��ϼ�Ϊ____��
��2������1�ijɷ�Ϊ________������2�ijɷ�Ϊ____��
��3��������l����ϡ�����ܽ�����ҺW���������Һ�н������ӵķ����� ��
��4���������е�Al2O3�����ڹ�ҵ�Ͽ�����ұ�������û�ѧ����ʽΪ
��5�������йع��ұ�����CrO42���ķ�ˮҪ����ѧ������ʹ��Ũ�Ƚ���5��0��10��7 mo1/L���²����ŷš���CrO42���ķ�ˮ����ͨ�����������ַ�����
�ٳ���������������Ա�������BaCrO4����
 ���ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С�� mol/L��������ˮ�������ܴﵽ�����ŷű���
���ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С�� mol/L��������ˮ�������ܴﵽ�����ŷű����ڻ�ԭ����
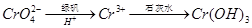 ��д������������ CrO42�����̷�����Һ�з�Ӧ�����ӷ���ʽ ��
��д������������ CrO42�����̷�����Һ�з�Ӧ�����ӷ���ʽ ����6��ij��Ч��ˮ������K2FeO4�õ��ģ���ҵ������ҺW��������غ���������Ϊԭ���Ʊ�K2FeO4���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��1��+3��1�֣�
��2��Fe2O3 Al��OH��3����1�֣�������������Ҳ�ɣ�
��3��ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ��죬֤����Fe3+������������Ҳ�ɣ���2�֣�
��4��2 Al2O3 4Al + 3O2����1�֣�
4Al + 3O2����1�֣�
��5����2��4��10��4��2�֣���CrO42����3Fe2+��8H+��Cr3+��3Fe3+��4H2O��3�֣�
��6��Fe2��SO4��3 + 3KClO + 10KOH = 2K2FeO4 + 3K2SO4 + 3KCl + 5H2O��3�֣�
��2��Fe2O3 Al��OH��3����1�֣�������������Ҳ�ɣ�
��3��ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ��죬֤����Fe3+������������Ҳ�ɣ���2�֣�
��4��2 Al2O3
 4Al + 3O2����1�֣�
4Al + 3O2����1�֣���5����2��4��10��4��2�֣���CrO42����3Fe2+��8H+��Cr3+��3Fe3+��4H2O��3�֣�
��6��Fe2��SO4��3 + 3KClO + 10KOH = 2K2FeO4 + 3K2SO4 + 3KCl + 5H2O��3�֣�
�����������1���ڸ�����Fe��CrO2��2�У�Fe�Ļ��ϼ�Ϊ+2������Cr�Ļ��ϼ�Ϊ+3��
��2�������ʵĸ��������ա�����ˮ��ֻ��������������ˮ����Ϊ������ȥ����������1�ijɷ���Fe2O3����ʱ��ҺΪNa2CrO4�� NaAlO2�Ļ��Һ��ͨ��CO2������NaAlO2��Ӧ����Al��OH��3����������2�ijɷ���Al��OH��3��
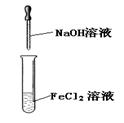
��3��������l����ϡ�����ܽ�����ҺW Fe2��SO4��3������Fe3+�ķ�����ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ��죬֤����Fe3+
��4����ҵ���õ�������������ķ������õ�������ѧ����ʽΪ2 Al2O3
 4Al + 3O2����
4Al + 3O2������5����Ksp��BaCrO4��=1��2��10��10��c��CrO42����<=5��0��10��7 mo1/L,����c��Ba2+��>=1��2��10��10/5��0��10��7=2��4��10��4 mo1/L, ������������ CrO42�����̷�FeSO4?7 H2O��Ӧ��CrO42�������������̷�����ԭ���������ӷ���ʽΪCrO42����3Fe2+��8H+��Cr3+��3Fe3+��4H2O
��6��W ΪFe2��SO4��3������ҺFe2��SO4��3��������غ���������Ϊԭ���Ʊ�K2FeO4���÷�Ӧ�Ļ�ѧ����ʽ��Fe2��SO4��3 + 3KClO + 10KOH = 2K2FeO4 + 3K2SO4 + 3KCl + 5H2O

��ϰ��ϵ�д�
�����Ŀ
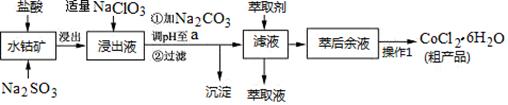
 N2O4��g�� ��H<0
N2O4��g�� ��H<0