��Ŀ����
������������Ԫ��֮һ,����ֲ���纣���������к��зḻ�ġ��Ի���̬��ʽ���ڵĵ�Ԫ�ء���ʵ������,�Ӻ�������ȡ������̺�ʵ��װ������:
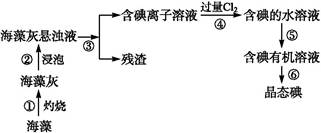
(1)ָ��������ȡ��Ĺ������й�ʵ�����������:�������������,���������������
(2)д������ܶ�Ӧ��Ӧ�����ӷ���ʽ:����������������������
(3)��ȡ��Ĺ�����,�ɹ�ѡ����л��Լ�������������(����)
A.�ƾ� B.���� C.���Ȼ�̼ D.��
(4)����ܳ��˼������Cl2,�������������ѡ����������(�����)��
A.Ũ���� B.H2O2��Һ C.KMnO4��Һ
������____________________________��
(5)Ϊ��ʹ������еĵ�����ת��Ϊ����л���Һ,����ɲ��������,ʵ���������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������Ʒ,��ȱ�ٵIJ�������������������
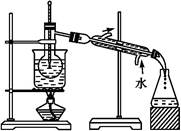
(6)�Ӻ�����л��ܼ�����ȡ��ͻ����л��ܼ�,����Ҫ��������ָ����ͼʵ��װ���д��ڵĴ���֮��:����

(1)ָ��������ȡ��Ĺ������й�ʵ�����������:�������������,���������������
(2)д������ܶ�Ӧ��Ӧ�����ӷ���ʽ:����������������������
(3)��ȡ��Ĺ�����,�ɹ�ѡ����л��Լ�������������(����)
A.�ƾ� B.���� C.���Ȼ�̼ D.��
(4)����ܳ��˼������Cl2,�������������ѡ����������(�����)��
A.Ũ���� B.H2O2��Һ C.KMnO4��Һ
������____________________________��
(5)Ϊ��ʹ������еĵ�����ת��Ϊ����л���Һ,����ɲ��������,ʵ���������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������Ʒ,��ȱ�ٵIJ�������������������
(6)�Ӻ�����л��ܼ�����ȡ��ͻ����л��ܼ�,����Ҫ��������ָ����ͼʵ��װ���д��ڵĴ���֮��:����

(1)���ˡ���ȡ����Һ(ÿ��1��)
(2)Cl2+2I- I2+2Cl-(2��)
I2+2Cl-(2��)
(3)CD(1��)
(4)B(1��)
������������ɫ������,�����������в�����������,Ҳ��������Ⱦ(2��)
(5)��Һ©������ͨ©��(2��)
(6)�¶ȼ�ˮ�����λ�ò���(2��)
(2)Cl2+2I-
 I2+2Cl-(2��)
I2+2Cl-(2��)(3)CD(1��)
(4)B(1��)
������������ɫ������,�����������в�����������,Ҳ��������Ⱦ(2��)
(5)��Һ©������ͨ©��(2��)
(6)�¶ȼ�ˮ�����λ�ò���(2��)
(1)������ͼ�п�֪�������ǹ���,����������ȡ����Һ��(2)Cl2�������Դ���I2,�ɷ�����ӦCl2+2I- I2+2Cl-��(3)������ȡԭ��,Ҫ�Ӻ����ˮ��Һ����ȡ��,��ѡ��ȡ��һ��Ҫ��ˮ�������ܻ��������,����������ȡ�����ܽ��Ҫ����ˮ�д�öࡣ��ѡ���оƾ����������ˮ���ܡ����C��D��ȷ��(4)������ѡ��������,��Ϊ������ɫ������,��Ӧ����������Ⱦ�������(5)��������ͼ����ÿһ����Ҫ��������ȱ����ͨ©���ͷ�Һ©����(6)���ʵ��װ�õĴ���,Ҫ��ʵ�������һ��顣��ͼʾװ�ÿɿ����¶ȼ�ˮ�����λ�ò��ԡ�
I2+2Cl-��(3)������ȡԭ��,Ҫ�Ӻ����ˮ��Һ����ȡ��,��ѡ��ȡ��һ��Ҫ��ˮ�������ܻ��������,����������ȡ�����ܽ��Ҫ����ˮ�д�öࡣ��ѡ���оƾ����������ˮ���ܡ����C��D��ȷ��(4)������ѡ��������,��Ϊ������ɫ������,��Ӧ����������Ⱦ�������(5)��������ͼ����ÿһ����Ҫ��������ȱ����ͨ©���ͷ�Һ©����(6)���ʵ��װ�õĴ���,Ҫ��ʵ�������һ��顣��ͼʾװ�ÿɿ����¶ȼ�ˮ�����λ�ò��ԡ�
 I2+2Cl-��(3)������ȡԭ��,Ҫ�Ӻ����ˮ��Һ����ȡ��,��ѡ��ȡ��һ��Ҫ��ˮ�������ܻ��������,����������ȡ�����ܽ��Ҫ����ˮ�д�öࡣ��ѡ���оƾ����������ˮ���ܡ����C��D��ȷ��(4)������ѡ��������,��Ϊ������ɫ������,��Ӧ����������Ⱦ�������(5)��������ͼ����ÿһ����Ҫ��������ȱ����ͨ©���ͷ�Һ©����(6)���ʵ��װ�õĴ���,Ҫ��ʵ�������һ��顣��ͼʾװ�ÿɿ����¶ȼ�ˮ�����λ�ò��ԡ�
I2+2Cl-��(3)������ȡԭ��,Ҫ�Ӻ����ˮ��Һ����ȡ��,��ѡ��ȡ��һ��Ҫ��ˮ�������ܻ��������,����������ȡ�����ܽ��Ҫ����ˮ�д�öࡣ��ѡ���оƾ����������ˮ���ܡ����C��D��ȷ��(4)������ѡ��������,��Ϊ������ɫ������,��Ӧ����������Ⱦ�������(5)��������ͼ����ÿһ����Ҫ��������ȱ����ͨ©���ͷ�Һ©����(6)���ʵ��װ�õĴ���,Ҫ��ʵ�������һ��顣��ͼʾװ�ÿɿ����¶ȼ�ˮ�����λ�ò��ԡ�
��ϰ��ϵ�д�
�����Ŀ


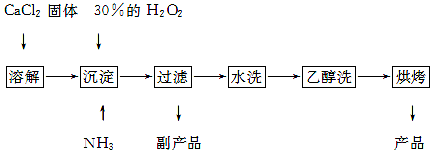
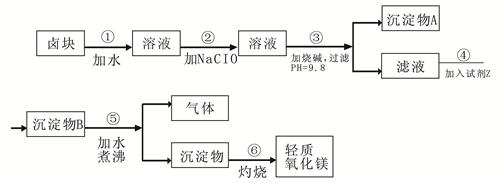
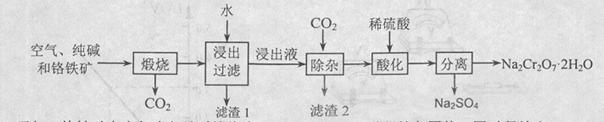
 2NaAlO2+CO2������ش�
2NaAlO2+CO2������ش� ���ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С�� mol/L��������ˮ�������ܴﵽ�����ŷű���
���ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С�� mol/L��������ˮ�������ܴﵽ�����ŷű���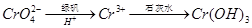 ��д������������ CrO42�����̷�����Һ�з�Ӧ�����ӷ���ʽ ��
��д������������ CrO42�����̷�����Һ�з�Ӧ�����ӷ���ʽ ��