��Ŀ����
2�������Ԫ�ذ�ԭ��������������Ϊ��ﮣ�Li�����ƣ�Na�����أ�K����﨣�Rb����藍�Cs�����գ�Fr��������Ԫ�أ�����ش���1�����³�ѹ�£���һ����﮵���Ͷ�뵽һ������ˮ�У����ȫ��Ӧ������1.6g���壬���˵õ�6.75g������100mL������Һ����õ��ij�����Һ�����ʵ����ʵ���Ũ��Ϊ5.3mol/L��
��2���ƼغϽ��������ⷴӦ�����ŷdz���Ҫ��Ӧ�ã�ȡ59.80g�ƼغϽ��һ�������Ȼ�̼��һ��������ǡ����ȫ��Ӧ�������5.40g�������ʯ��ĩ������ƼغϽ�����Ԫ�غͼ�Ԫ�ص�������Ϊ1��3��
���� ��1�����ɵ�����Ϊ���⣬����n=$\frac{m}{M}$����������ʵ������ٸ��ݷ�Ӧ��ϵʽ���������﮵����ʵ������Ӷ��ó�����������﮵������ʵ������ټ��������������﮳��������ʵ���������Եõ���Һ��������﮵����ʵ������ٸ���c=$\frac{n}{V}$�õ��ij�����Һ�����ʵ����ʵ���Ũ�ȣ�
��2��������Ӧ�У�CCl4+4Na$\stackrel{һ��������}{��}$C�����ʯ��+4NaCl��CCl4+4K$\stackrel{һ��������}{��}$C�����ʯ��+4KCl����������ƺͼص����ʵ�����Ȼ��ֱ���ݺϽ������������ɽ��ʯ������ʽ���㣮
��� �⣺��1��6.75g����Ϊ������ﮣ����ʵ���Ϊ��$\frac{6.75g}{25g/mol}$=0.27mol��
1.6g��������ʵ���Ϊ��$\frac{1.6g}{4g/mol}$=0.4mol����Ӧ����﮵������ʵ���Ϊ��0.4mol��2=0.8mol��
����Һ��������﮵����ʵ���Ϊ��0.8mol-0.27mol=0.53mol��
���Եõ��ij�����Һ�����ʵ����ʵ���Ũ��Ϊ��$\frac{0.53mol}{0.1L}$=5.3mol/L��
�ʴ�Ϊ��5.3mol/L��
��2���ơ��������Ȼ�̼��Ӧ�ķ���ʽΪ��CCl4+4Na$\stackrel{һ��������}{��}$C�����ʯ��+4NaCl��CCl4+4K$\stackrel{һ��������}{��}$C�����ʯ��+4KCl��
��Ͻ����ơ��ص����ʵ����ֱ�Ϊx��y��
������������ɵã���23x+39y=59.80g��
���ݷ�Ӧ����ʽ��֪�����ɽ��ʯ������Ϊ����12����x+y����$\frac{1}{4}$=5.40g��
�����٢ڽ�ã�x=0.65��y=1.15��
��Ͻ����ơ��ص�������Ϊ����23g/mol��0.65mol������39g/mol��1.15mol��=1��3��
�ʴ�Ϊ��1��3��
���� ���⿼���˻���ﷴӦ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ������Ӧԭ��Ϊ���ؼ���ע�����������غ㶨���ڻ�ѧ�����е�Ӧ�ã���1������Ҫ��ȷ������﮷�Ϊ�����֣�һ�������ɳ�������һ����Ϊ��Һ�е����ʣ�Ϊ�״��㣮

| A�� | Ca2++ClO-+SO2+H2O��CaSO4��+Cl-+2H+ | |
| B�� | Ca2++2ClO-+2SO2+2H2O��CaSO4��+2Cl-+2H++SO42- | |
| C�� | Ca2++3ClO-+3SO2+3H2O��CaSO4��+3Cl-+6H++2SO42- | |
| D�� | Ca2++3ClO-+SO2+H2O��CaSO4��+2HClO+Cl- |
| Ԫ���� | ����Ԫ�� | ����Ԫ���������͵ı�� |
| ��1��S��N��Na��Mg | N | �� |
| ��2��P��Sb��Si��As | Si | �� |
| ��3��Rb��B��Br��Fe | Fe | �� |
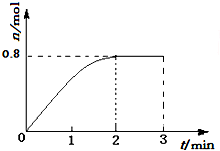 һ�������£���3molA��1molB�����������ڹ̶��ݻ�Ϊ2L���ܱ������У��������·�Ӧ��3A��g��+B��g��?C��g��+2D��s����2minĩ�÷�Ӧ�ﵽƽ�⣬����D�����ʵ�����ͼ�������ж���ȷ���ǣ�������
һ�������£���3molA��1molB�����������ڹ̶��ݻ�Ϊ2L���ܱ������У��������·�Ӧ��3A��g��+B��g��?C��g��+2D��s����2minĩ�÷�Ӧ�ﵽƽ�⣬����D�����ʵ�����ͼ�������ж���ȷ���ǣ�������| A�� | �����������ܶȲ��ٸı�ʱ���÷�Ӧ��һ���ﵽƽ��״̬ | |
| B�� | 2min��ѹ��ʹ����Ӧ���ʼӿ죬�淴Ӧ���ʱ�����ƽ�������ƶ� | |
| C�� | ��Ӧ������A��B��ת����֮��Ϊ3��1 | |
| D�� | �������´˷�Ӧ�Ļ�ѧƽ�ⳣ������ֵԼΪ0.91 |
 ��BA2�Ľṹʽ�ǣ�S=C=S��
��BA2�Ľṹʽ�ǣ�S=C=S�� Ԫ�����ڱ��е� VIIA��Ԫ�صĵ��ʼ��仯�������;�㷺��
Ԫ�����ڱ��е� VIIA��Ԫ�صĵ��ʼ��仯�������;�㷺��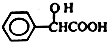 ����ҵ�ϱ������������A�������²���ϳɵõ���
����ҵ�ϱ������������A�������²���ϳɵõ���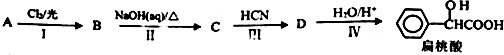
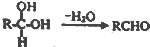
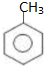 ���������к��������������ǻ����Ȼ���
���������к��������������ǻ����Ȼ��� ��
��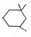 ����Ũ���������·�Ӧ���ɣ���д����Ӧ�Ļ�ѧ����ʽ
����Ũ���������·�Ӧ���ɣ���д����Ӧ�Ļ�ѧ����ʽ
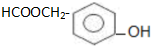 ��
��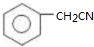 ��Ϊԭ�Ϻϳɱ������ͬ���칹��-���ǻ������ᣨ
��Ϊԭ�Ϻϳɱ������ͬ���칹��-���ǻ������ᣨ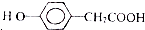 ���ĺ���·�ߣ�
���ĺ���·�ߣ�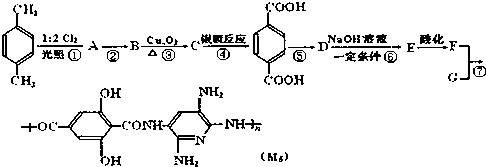
 ��
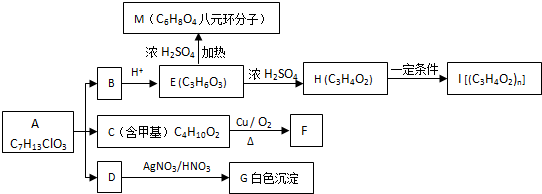
�� ��F�ĺ��������ŵ��������Ȼ������ӣ��ǻ���
��F�ĺ��������ŵ��������Ȼ������ӣ��ǻ��� ��
��

 +O2$��_{��}^{Cu}$
+O2$��_{��}^{Cu}$ +2H2O����E��M��2HOCH2CH2COOH $��_{��}^{ŨH_{2}SO_{4}}$
+2H2O����E��M��2HOCH2CH2COOH $��_{��}^{ŨH_{2}SO_{4}}$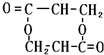 +2H2O��
+2H2O��