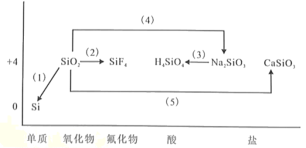
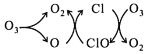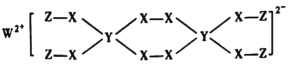��Ŀ����
����Ŀ��̼���γɻ�������������Ԫ�أ��䵥�ʼ��仯���������������������Ҫ��Դ���ʡ���ش��������⣺
(1)�л���M����̫������տ�ת����N��ת���������£�
 ��
��
��H��88.6 kJ/mol��M��N��ȣ����ȶ�����________��
(2)��֪CH3OH(l)��ȼ����Ϊ-726.5 kJ��mol��1��CH3OH(l)��1/2O2(g)===CO2(g)��2H2(g)����H����a kJ��mol��1����a________726.5(���������������)��
(3)ʹCl2��H2O(g)ͨ�����ȵ�̿�㣬����HCl��CO2������1 mol Cl2���뷴Ӧʱ�ͷų�145 kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��_______________________________��
(4)����͵�������ı������������ʡ���ʯī�����ۺͶ������Ѱ�һ����������ڸ��������գ��������ʿ������²��ϣ�4Al(s)��3TiO2(s)��3C(s)===2Al2O3(s)��3TiC(s)����H����1176 kJ��mol��1����Ӧ�����У�ÿת��1 mol���ӷų�������Ϊ________��
���𰸡�M �� 2Cl2(g)��2H2O(g)��C(s)===4HCl(g)��CO2(g)��H����290 kJ��mol��1 98 kJ
��������
���⿼�黯ѧ�������ܵ��ۺ����ã���Ҫ�ӻ�ѧ��Ӧ�ȵļ��㣬�Լ������Ȼ�ѧ��Ӧ����ʽ�����������仯��˼·���н��
��1��Mת��ΪN�����ȷ�Ӧ������N�������ߣ����ȶ���
��2���״�ȼ������CO2(g����H2(g�����ڲ���ȫȼ�գ��ų��������٣���a<238.6��
��3����1mol Cl2���뷴Ӧʱ�ͷų�145kJ������2mol������Ӧ����290kJ��ע���ʾۼ�״̬�Ͷ�Ӧ��Ӧ�ʱ�д���Ȼ�ѧ����ʽ��
��4��������Ӧ��ת��12�����ӣ���ÿת��1mol���ӷų�������Ϊ1 176kJ��12=98kJ��
��1���л���M����̫������տ�ת����N����H = +88.6kJmol-1���ù��������ȷ�Ӧ��N����ת��ΪM���Ƿ��ȷ�Ӧ�����ݷ�Ӧ�������Խ��Խ�ȶ�����֪M�ȶ���
�ʴ�Ϊ��M��
��2��ȼ������1mol������ȫȼ�������ȶ�������ų����������״�ȼ������CO2(g)��H2(g)���ڲ���ȫȼ�գ��ų�������С��ȼ���ȣ�
�ʴ�Ϊ��<��
��3����1 mol Cl2���뷴Ӧʱ�ͷų�145kJ������2 mol������Ӧ����290 kJ����Ӧ���Ȼ�ѧ����ʽΪ��2Cl2(g) + 2H2O(g) + C(s) �T 4HCl(g) + CO2(g) ��H = -290 kJmol-1��
�ʴ�Ϊ��2Cl2(g) + 2H2O(g) + C(g) �T 4HCl(g) + CO2(g) ��H = -290kJmol-1��
��4��4Al(g) + 3TiO2(g) + 3C(g) �T 2Al2O3(g) + 3TiC(g) ��H = -1176 kJmol-1��ת��12mol���ӷ���1176KJ����Ӧ�����У�ÿת��1mol���ӷ���98kJ��
�ʴ�Ϊ��98kJ��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ij��ѧ�о���ѧϰС���ͬѧ�������۷�������Ϊ��NO2���ܻ���������ͭ����������������ͼ��ʾװ����֤NO2��������(�г�װ����ʡ��)��
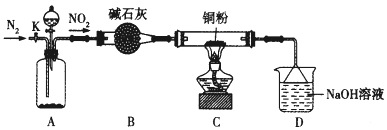
(1)װ��A~C����һ���������ķ�����_____________��A�з�����Ӧ�������Լ�ͨ����________��
(2)��ȼC���ƾ��ƱȽϺ��ʵ�ʱ����_____________����C�й���ȫ����ɺ�ɫ��ͬʱ���ɶԻ�������Ⱦ�����壬д��C�з�Ӧ�Ļ�ѧ����ʽ��______________��װ��D����;��____________��
(3)����K���������ʵ����ɫ��ѧʵ��ΪĿ�ģ���˵��K��ʹ�÷���______________��(����ʹ��ʱ��������)��
(4)ʵ���������C�еĹ�����������ĺ�ɫ��ĩ��������������ͬѧ��Ϊ�ú�ɫ��ĩ��Cu2O����ͬѧ��Ϊ�ú�ɫ��ĩ��![]() ����ͬѧ��Ϊ�ú�ɫ��ĩ��Cu��Cu2O�Ļ����������ϣ�Cu��Cu2O��CuO�IJ����������£�
����ͬѧ��Ϊ�ú�ɫ��ĩ��Cu��Cu2O�Ļ����������ϣ�Cu��Cu2O��CuO�IJ����������£�
ϡ���� | ��ˮ | |
Cu2O | ����Cu��Cu2+ | ������ɫ[Cu(NH3)2]2+ |
CuO | ����Cu2+ | ������ɫ[Cu(NH3)4]2+ |
Cu | --- | --- |
�����һ����ʵ��֤����ͬѧ�Ŀ����Ƿ���ȷ��________________��
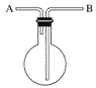
����Ŀ��ij��ȤС���Ʊ�һ��������������.ȡ3mL��ˮ�Ҵ�,2mLŨ����,2mL���������ʵ�飬��5mL����̼������Һ�ռ����
I.ʵ��װ����ͼ��ʾ
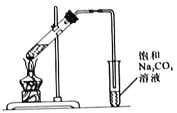
��1���Ʊ����������Ļ�ѧ����ʽΪ_______________��
��2��Ũ�����������_______________��
��3�������ܵ�������_______________��
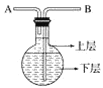
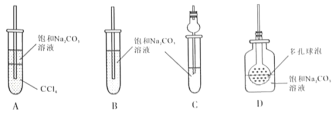
��4������װ�û���ѡ����ͼ�е�___________��(�����).
��.��ͬѧ�ú��з�̪�ı���̼������Һ(�ʼ���)�ռ�����������ֺ�ɫѸ����ȥ.
��ͬѧ��Ϊ�������������к���̼����.��ͬѧͨ���������ϲ���������ʵ�飬֤����ͬѧ���Ʋ��Ǵ���ġ�
��֪����̪������ˮ���������л��ܼ�����̪�Լ��Ƿ�̪���Ҵ���Һ.
ʵ��i��ȡ����²���ɫҺ�壬�ֳ����ݣ��ֱ��������ʵ��
��� | ʵ����� | ʵ������ | ���� |
1 | �μӼ�����̪�Լ� | ��Һ �� (��������������������) | ̼���Ʋ�δ��������ȫ�кͣ����д���ʣ�� |
2 | ����������Һ | �д������ݲ��� |
ʵ��ii.ȡ����ϲ�Һ�壬���� �� ��Һ�������ֳ���dz��ɫ�����÷ֲ���ɫ��ʧ��
ʵ��iii��ȡ5mL����̼������Һ�����뼸�η�̪�Լ����ټ���3mL��������(��������)����Һ�ȱ�죬���ɫ��ʧ���ش���������
��5���������ʵ�飺��_______________����_______________��
��6�����ʵ��ii��ʵ��iii�����ɵó��Ľ�����_______________��
��7��ʵ��iii��ʵ��Ŀ����_______________��