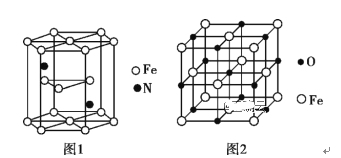
��Ŀ����
����Ŀ��NVCO{��ѧʽ�ɱ�ʾΪ(NH4)a[(VO)b(CO3)c(OH)d]��10H2O}��������ȡVO2��ʵ���ҿ���V2O5��N2H4��2HCl��NH4HCO3Ϊԭ���Ʊ�NVCO��
(1)ԭ��NH4HCO3��HCO3-ˮ������ӷ���ʽΪ____________��
(2) N2H4��2HCl��N2H4�������Ρ���֪N2H4��ˮ�еĵ��뷽ʽ��NH3���ƣ�25 ��ʱ��K1=9.55��10-7�����¶��£���ӦN2H4+H��![]() N2H5+��ƽ�ⳣ��K=________(����ֵ)��
N2H5+��ƽ�ⳣ��K=________(����ֵ)��
(3)Ϊȷ��NVCO����ɣ���������ʵ�飺
�ٳ�ȡ2.130 g��Ʒ������NaOH��ַ�Ӧ������NH3 0.224 L(�ѻ���ɱ�״����)��
����ȡһ������Ʒ�ڵ������м��ȣ���Ʒ�Ĺ��������(������Ʒ��ʣ������/������Ʒ����ʼ������100%)���¶ȵı仯����ͼ��ʾ(�ֽ�����и�Ԫ�صĻ��ϼ۲���)��
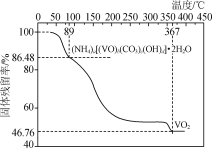
��������ʵ�����ݼ���ȷ��NVCO�Ļ�ѧʽ(д���������)________________��
���𰸡�HCO3-+H2O![]() H2CO3��OH- 9.55��107 (NH4)5[(VO)6(CO3)4(OH)9]��10H2O
H2CO3��OH- 9.55��107 (NH4)5[(VO)6(CO3)4(OH)9]��10H2O
��������
(1)HCO3-ˮ������H2CO3��OH-��
(2)����Ϊ��Ԫ�����ˮ�еĵ��뷽ʽ�백���ơ�������һ�����뷽��ʽΪN2H4+H2O![]() N2H5++OH-����ӦN2H4+H��
N2H5++OH-����ӦN2H4+H��![]() N2H5+��ƽ�ⳣ��K=
N2H5+��ƽ�ⳣ��K=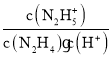 =
=![]() ��
��
(3)��NVCO��Ħ������ΪM g/mol����![]() =0.864 8���ɵ�M=1 065����
=0.864 8���ɵ�M=1 065����![]() �ɵ�b=6����
�ɵ�b=6����![]() ��a=n(NH3)=
��a=n(NH3)=![]() =0.01mol�����a=5����������ΪVO2����VOΪ+2�ۣ�2c+d=5��1+2��6=17���ɻ��������Է�������18a+67b+60c+17d+180=1065��֪60c+17d=393�����c=4��d=9����a=5��b=6��c=4��d=9���뻯ѧʽ����ʽ���ɵû�ѧʽ��
=0.01mol�����a=5����������ΪVO2����VOΪ+2�ۣ�2c+d=5��1+2��6=17���ɻ��������Է�������18a+67b+60c+17d+180=1065��֪60c+17d=393�����c=4��d=9����a=5��b=6��c=4��d=9���뻯ѧʽ����ʽ���ɵû�ѧʽ��
(1)HCO3-ˮ������H2CO3��OH-��ˮ�ⷴӦ���ӷ���ʽΪHCO3-+H2O![]() H2CO3+OH-��
H2CO3+OH-��
(2)����Ϊ��Ԫ�����ˮ�еĵ��뷽ʽ�백���ơ�������һ�����뷽��ʽΪN2H4+H2O![]() N2H5++OH-����ӦN2H4+H��
N2H5++OH-����ӦN2H4+H��![]() N2H5+��ƽ�ⳣ��K=
N2H5+��ƽ�ⳣ��K=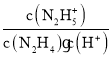 =
=![]()
![]() =9.55��107��
=9.55��107��
(3)��NVCO��Ħ������ΪM g/mol����![]() =0.864 8���ɵ�M=1 065����
=0.864 8���ɵ�M=1 065����![]() �ɵ�b=6����
�ɵ�b=6����![]() ��a=n(NH3)=
��a=n(NH3)=![]() =0.01mol�����a=5����������ΪVO2����VOΪ+2�ۣ�2c+d=5��1+2��6=17���ɻ��������Է�������18a+67b+60c+17d+180=1065��֪60c+17d=393�����c=4��d=9����a=5��b=6��c=4��d=9���뻯ѧʽ����ʽ���ɵû�ѧʽΪ(NH4)5[(VO)6(CO3)4(OH)9]��10H2O��
=0.01mol�����a=5����������ΪVO2����VOΪ+2�ۣ�2c+d=5��1+2��6=17���ɻ��������Է�������18a+67b+60c+17d+180=1065��֪60c+17d=393�����c=4��d=9����a=5��b=6��c=4��d=9���뻯ѧʽ����ʽ���ɵû�ѧʽΪ(NH4)5[(VO)6(CO3)4(OH)9]��10H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���������ף�����Ũ����ͻ��ý�����Ӧ�����У���������Ũ�ȵĽ��ͣ������ɵIJ�������4����2����3�۵��Ļ����
��FeSO4 + NO![]() Fe(NO)SO4(��ɫ)����H��0��
Fe(NO)SO4(��ɫ)����H��0��
��NO2��NO���ܱ�����KMnO4��Һ�������ա�
�װ�����ͼ��ʾ��ʵ��װ�ý���ʵ�飺
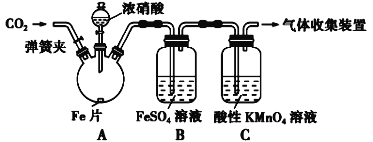
ʵ������������¼�����ʾ��
ʵ����� | ʵ������ |
���ɼУ�ͨ��һ��ʱ��CO2���رյ��ɼ� | |
��Һ©����������Ũ���Ỻ��������ƿ�У��رջ��� | ���������� |
������ƿ����Ӧ��ʼ��ֹͣ���� | ��A���к���ɫ���������һ��ʱ���������ɫ��dz��B����Һ����ɫ��C����Һ��ɫ��dz�� �ڷ�Ӧֹͣ��A������ʣ�� |
��ش��������⣺
�� ����ǰ������ƿ�е���Ũ����û�����������ԭ����________________________________
�� �����Ƿ����ɣ�3�۵��Ļ����Ӧ���е�ʵ�������________________________________
�� ��ȡ����B����Һ�����ȣ�ʵ��������____________________________________________�����û�ѧƽ��ԭ������ԭ��________________________________________________________�������ݸ�����ó����ۣ��������ᷴӦ��NO���ɡ�
������Ϊ�ó�A����NO���ɵ�֤�ݲ��㡣Ϊ��ȡ�����֤�ݣ����Բ��ø�װ�úͲ������ж���ʵ�飬�������ĸı���____________________________��֤����NO���ɵ�ʵ��������________________________________________________
����Ŀ����������ʵ��������������ó��Ľ�����ȷ����(����)
ѡ�� | ʵ����������� | ���� |
A | ��ʢ����������ϡ��Һ���Թ�����μ��뱥����ˮ��������ɫ���� | ������Br2��Ӧ����2��4��6���屽�� |
B | ��H2O2��Һ�е���NaClO��Һ��������ɫ���� | H2O2���������� |
C | ��FeCl3��Һ�е�������KI��Һ���ټ���KSCN��Һ����Һ��� | Fe3����I���ķ�Ӧ���п����� |
D | �������еμ�����Ũ���ᣬ���裬���DZ�ڣ�������ɣ�������ɶ�ĺ���״ | Ũ���������ˮ�Ժ������� |
A. AB. BC. CD. D