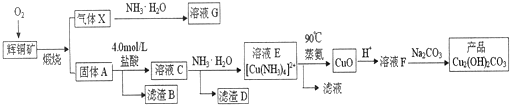
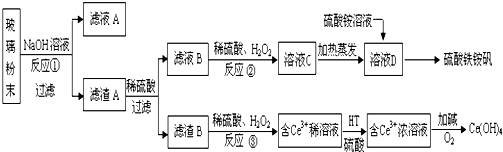
��Ŀ����
10��Ԫ�ظ����仯���﹤ҵ��;�㷺������+6�۸�����ˮ����������Ƴ������Ķ�ͭ��ˮ����������һ������Cr2O72-�������÷�ˮ����ֱ�ӳ�������ԭ��������I��ֱ�ӳ�����
��1����֪������ˮ�д�����ƽ�⣺Cr2O72-+H2O?2CrO42-+2H+����ʵ�ʹ�ҵ�����У����������BaCl2��Һ֮ǰ��Ҫ����һ������NaOH�����������ڳ��������ɣ������ɳ����Ļ�ѧʽΪBaCrO4
��ԭ������
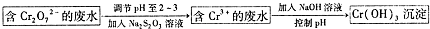
��2��������Һ�п��Դ�������������Na2S2O3��Һ����AD����ѡ����ţ���
A��FeSO4��Һ B��ŨH2SO4 C������KMnO4 D��Na2SO3��Һ
��3�����������У�ÿ����0.1mol Na2S2O3ת��0.8mol e-������Na2S2O3��Һʱ������Ӧ�����ӷ���ʽΪ3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
��4��Cr��OH��3�Ļ�ѧ������Al��OH��3���ƣ����������������м���NaOH��ҺʱҪ������Һ��PH���ܹ��ߣ�ԭ��������ӷ���ʽ��ʾ��Cr��OH��3+OH-=CrO2-+2H2O
��5��ʵ�ʹ�ҵ��������ʱ�����������ӽ�����֬�����ⶨ��������Һ��Cr3+�ĺ�������ԭ����Mn++nNaR�TnNa++MRn������NaRΪ�����ӽ�����֬��Mn+ΪҪ�ⶨ�����ӣ�
�������ӽ�����֬��ԭ����֮һ�Ǿ۱���ϩ���䵥��Ϊ����ϩ��
 CH=CH2�����۱���ϩ�Ļ�ѧʽΪ
CH=CH2�����۱���ϩ�Ļ�ѧʽΪ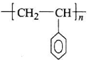 ��
����ij�βⶨ�����У���pH=5�ķ�ˮ���������ӽ�����֬�����Һ��Na+�Ƚ���ǰ������4.6��10-2g•L-1�����������Cr��OH��3��ksp��ֵΪ6.7��10-31��
���� ��1�����������Ӻ������ӷ�Ӧ����ˮ����ʹƽ��Cr2O72-+H2O?2CrO42-+2H+���ƣ������ڸ�Ԫ��ת��Ϊ���ᱵ��BaCrO4��������
��Cr2O72-���ӵķ�ˮ����Na2S2O3��Һ������ҺPH=2-3����ԭ�ظ�������ӵõ�Cr3+���ӵ���Һ����������������Һ������ҺPH����Cr��OH��3��
��2�����Դ�������������Na2S2O3��Һ����Ҫ���л�ԭ�ԣ��ܻ�ԭ�ظ�������ӣ�
��3��ÿ����0.1mol Na2S2O3ת��0.8mol e-��Na2S2O3 ��2SO42-��8e-��Cr2O72-��2Cr3+��6e-������������ԭ��Ӧ�����غ������ƽ��д������ԭ��Ӧ�����ӷ���ʽ��
��4��Cr��OH��3�Ļ�ѧ������Al��OH��3���ƣ���ʾ���ԣ������ܽ���ǿ�ᡢǿ����Һ�У�
��5���پ۱���ϩ�DZ���ϩһ�������·����ļӳɾۺϷ�Ӧ�������˸߷��ӻ�����۱���ϩ��
�������ӽ�����֬�����ⶨ��������Һ��Cr3+�ĺ�������ԭ����Mn++nNaR�TnNa++MRn������NaRΪ�����ӽ�����֬��Mn+ΪҪ�ⶨ�����ӣ����㱻����������Cr3+Ũ�ȣ���pH=5�ķ�ˮ������������Ũ��Ϊ10-9mol/L������ܶȻ������������Ksp��
��� �⣺��1��������ˮ�д�����ƽ�⣺Cr2O72-+H2O?2CrO42-+2H+����ʵ�ʹ�ҵ�����У����������BaCl2��Һ֮ǰ��Ҫ����һ������NaOH�����������ڳ��������ɣ����������Ӻ������ӷ�Ӧ����ˮ����ʹƽ��������У������ڸ�Ԫ��ת��Ϊ���ᱵ�����������ɳ����Ļ�ѧʽΪ��BaCrO4��
�ʴ�Ϊ��BaCrO4��
��2�����Դ�������������Na2S2O3��Һ����Ҫ���л�ԭ�ԣ��ܻ�ԭ�ظ�������ӣ�
A��FeSO4��Һ���������Ӿ��л�ԭ�ԣ����Ի�ԭCr2O72-���ӣ���A���ϣ�
B��ŨH2SO4 ����ǿ�����ԣ����ܱ��ֻ�ԭ�ԣ����ܻ�ԭCr2O72-����B�����ϣ�
C������KMnO4 ��ǿ���������ܻ�ԭCr2O72-����C�����ϣ�
D��Na2SO3��Һ������������Ӿ��л�ԭ�ԣ����Ի�ԭCr2O72-����D���ϣ�
�ʴ�Ϊ��AD��
��3��ÿ����0.1mol Na2S2O3ת��0.8mol e-��Na2S2O3 ��2SO42-��8e-��Cr2O72-��2Cr3+��6e-������������ԭ��Ӧ�����غ���ƽ��д��3Na2S2O3 ��6SO42-��24e-��4Cr2O72-��8Cr3+��24e-���õ���������ԭ��Ӧ�����ӷ���ʽΪ��3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
�ʴ�Ϊ��3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
��4�����������������м���NaOH��ҺʱҪ������Һ��PH���ܹ��ߣ�Cr��OH��3�Ļ�ѧ������Al��OH��3���ƣ�Cr��OH��3�����ܽ��ڹ���������������Һ�У��������ӷ���ʽ��ʾԭ��Ϊ��Cr��OH��3+OH-=CrO2-+2H2O��
�ʴ�Ϊ��Cr��OH��3+OH-=CrO2-+2H2O��
����5���پ۱���ϩ�DZ���ϩһ�������·����ļӳɾۺϷ�Ӧ�������˸߷��ӻ�����۱���ϩ���䵥��Ϊ����ϩ�� CH=CH2������ӦΪ��n
CH=CH2������ӦΪ��n CH=CH2��
CH=CH2�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
�������ӽ�����֬�����ⶨ��������Һ��Cr3+�ĺ�������ԭ����Cr3++3NaR�T3Na++CrR3������NaRΪ�����ӽ�����֬��Mn+ΪҪ�ⶨ������Cr3+��Na+�Ƚ���ǰ������4.6��10-2g•L-1�����ʵ���Ũ��=$\frac{4.6}{23}��1{0}^{-2}$mol/L=2��10-3mol/L�����㱻����������Cr3+Ũ��=$\frac{1}{3}$��2��10-3mol/L��Cr��OH��3��s��?3c��OH-��+c��Cr3+��
��pH=5�ķ�ˮ���������ӽ�����֬����Һ��c��OH-��=10-9mol/L��Ksp=c3��OH-��c��Cr3+��=[10-9]3��$\frac{1}{3}$��2��10-3mol/L=6.7��10-31��
�ʴ�Ϊ��6.7��10-31��
���� ���⿼�������ʷ����ᴿ�ķ������̷��������ӷ���ʽ��д���ܶȻ������ļ���Ӧ�ã���Ҫ��������ԭ��Ӧ������Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

I������NO2��һ�ַ��������ü������ԭNO2��
CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=-574kJ��mol-1
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2
CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H3=-867kJ��mol
���H2=-1160kJ•mol-1
��ʯȼ�ϵ�ȼ�գ����������ʯ��ұ������������������в�����SO2�Ǵ�����SO2����Ҫ��Դ��
��úת��Ϊ˭ÿ���ǽ�úת��Ϊ�ᾧȼ�ϵķ���֮һ����ӦΪ��C��s��+H2O��g��?CO��g��+H2��g��
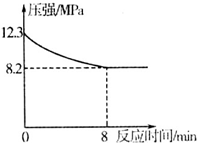
һ���¶��£���1.0L�ܱ������з���1molC��s����1molH2O��g�����з�Ӧ����Ӧʱ�䣨t����������������ѹǿƽ��p�������ݼ��±�
| ʱ��t/h | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 | 30 |
| ��ѹǿp/100kPa | 4.56 | 5.14 | 5.87 | 6.30 | 7.24 | 8.16 | 8.18 | 8.20 | 8.20 |
��1��������Щѡ�����˵���ÿ��淴Ӧ�Ѵ�ƽ��״̬AD
A�����������ܶȲ��ٷ����ı�
B������1molH2O��g����ͬʱ����1molH2
C����H����
D��V����CO��=V����H2��
��2������ѹǿP����ʼѹǿP0��ʾ��Ӧ��ϵ�������ʵ���n�ܣ�n��=$\frac{P}{{P}_{0}}$mol���ɱ������ݼ���ﵽƽ��ʱ����Ӧ��H2O��g����ת���ʦ�=79.82%����ȷ��С�����ڶ�λ��
��3����ѭ�����ղ���������SO2���ͻ�����Ⱦ��ͬʱ����ֵ�ð����������������£�
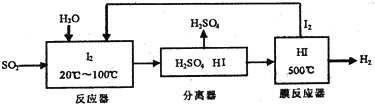
���û�ѧ����ʽ��ʾ�������̷������ܷ�ӦSO2+2H2O=H2SO4+H2
���û�ѧƽ���ƶ���ԭ����������HI�ֽⷴӦ��ʹ��Ĥ��Ӧ�������H��Ŀ���ǽ����������Ũ�ȣ�ʹƽ�����������ƶ�
��1����1.0molCH4��2.0molH2O��g��ͨ���ݻ�Ϊ100L�ķ�Ӧ������һ�������·�����Ӧ��
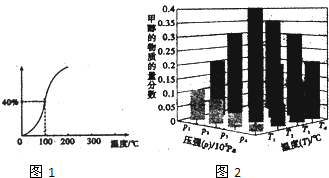
CH4��g��+H2O��g��?CO��g��+3H2��g�������һ����ѹǿ��CH4��ƽ��ת�������¶ȵĹ�ϵ��ͼ1.100��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ7.2��10-5
��2����һ���¶Ⱥ�ѹǿ�����·����˷�Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H��O����Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ2��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж�ȷ����CD������ţ�
A��P3��P2��T3��T2 B��P2��P4��T4��T2 C��P1��P3��T1��T3 D��P1��P4��T1��T4
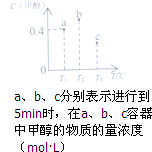 ��֪CO��H2��һ�������ºϳɼ״��ķ�ӦΪCO��g��+2H2��g��?CH3OH��g���������ݻ��̶�����ȵ�a��b��c�����ܱ������зֱ����1molCO��2molH2�Ļ�����壬�����¶Ƚ��з�Ӧ�����������ݵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
��֪CO��H2��һ�������ºϳɼ״��ķ�ӦΪCO��g��+2H2��g��?CH3OH��g���������ݻ��̶�����ȵ�a��b��c�����ܱ������зֱ����1molCO��2molH2�Ļ�����壬�����¶Ƚ��з�Ӧ�����������ݵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | a�����У�0��5min��Ӧ����v��H2��=0.08mol•L-1•min-1 | |
| B�� | ��Ӧ���е�5minʱ��b������v��=v�� | |
| C�� | ������������ʱ����ѹ�ɽ�b�е�״̬ת���c�е�״̬ | |
| D�� | �ﵽƽ��ʱa��b��c�����е�ת����Ϊb��a��c |
| A�� | KNO3 | B�� | CO��NH2��2 | C�� | Ca3��PO4��2 | D�� | K2SO4 |
 ��
��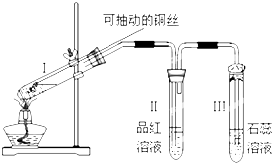
 ��
�� �о�̼���仯��������ʶ��ڿ��С��������������Ҫ���壮
�о�̼���仯��������ʶ��ڿ��С��������������Ҫ���壮