��Ŀ����
A��B��C��D��ԭ��������С��20������Ԫ�أ�A��Bͬ���壬�����γ�BA3�ͷ��ӣ�B��C��D���γɵļ����ӵ��Ӳ�ṹ��ͬ����B��C��D���Ӱ뾶���μ�С���ݴ˻ش��������⣺
��1��DԪ����Ԫ�����ڱ��е�λ���� ��
��2��B��D���γɵ���ͨ���������ʽΪ ��
��3����C���ʵ�ˮ��Һ�μӵ�B��D���γɻ������ˮ��Һ�У���������ɫ�������䷴Ӧ�Ļ�ѧ����ʽΪ ��
��4��ʵ�������ȥBA2���壬���������Լ��е� ������ĸ����
A�����Ը��������Һ B��ŨH2SO4 C��NaOH��Һ D��Ʒ����Һ��
��5����A��B��D����Ԫ������ɵ�ij����ˮ��Һ�Լ��ԣ���ԭ���ǣ������ӷ���ʽ��ʾ�� ��
��1��DԪ����Ԫ�����ڱ��е�λ����
��2��B��D���γɵ���ͨ���������ʽΪ
��3����C���ʵ�ˮ��Һ�μӵ�B��D���γɻ������ˮ��Һ�У���������ɫ�������䷴Ӧ�Ļ�ѧ����ʽΪ
��4��ʵ�������ȥBA2���壬���������Լ��е�
A�����Ը��������Һ B��ŨH2SO4 C��NaOH��Һ D��Ʒ����Һ��
��5����A��B��D����Ԫ������ɵ�ij����ˮ��Һ�Լ��ԣ���ԭ���ǣ������ӷ���ʽ��ʾ��
������A��B��C��D��ԭ��������С��20������Ԫ�أ�A��Bͬ���壬�����γ�BA3�ͷ��ӣ���AΪ��Ԫ�ء�BΪ��Ԫ�أ�B��C��D���γɵļ����ӵ��Ӳ�ṹ��ͬ�����������Ϊ18����B��C��D���Ӱ뾶���μ�С����ԭ������D��C��B�����ԭ��������֪��DΪK��CΪCl���ݴ˽��
����⣺A��B��C��D��ԭ��������С��20������Ԫ�أ�A��Bͬ���壬�����γ�BA3�ͷ��ӣ���AΪ��Ԫ�ء�BΪ��Ԫ�أ�B��C��D���γɵļ����ӵ��Ӳ�ṹ��ͬ�����������Ϊ18����B��C��D���Ӱ뾶���μ�С����ԭ������D��C��B�����ԭ��������֪��DΪK��CΪCl��
��1��DΪKԪ�أ���Ԫ�����ڱ��е�λ���ǣ��������ڵڢ�A�壬�ʴ�Ϊ���������ڵڢ�A�壻
��2��B��D���γɵ���ͨ������ΪK2O������ʽΪ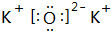 ���ʴ�Ϊ��
���ʴ�Ϊ��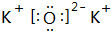 ��
��
��3����������ˮ��Һ�μӵ�K2S��ˮ��Һ�У���������ɫ����������������������ԭ��ͬʱ����KCl����Ӧ�Ļ�ѧ����ʽΪ��Cl2+K2S=2KCl+S�����ʴ�Ϊ��Cl2+K2S=2KCl+S����
��4��ʵ�������ȥSO2���壬����������л�ԭ�ԣ����������Ը������������ȥ�������������������������������Һ���գ�Ũ��������ն�������Ʒ����Һ���Լ����������ѡAC��
��5����A��B��D����Ԫ������ɵ�ij����ˮ��Һ�Լ��ԣ�����Ϊ�������ƣ���Һ��SO32-ˮ��SO32-+H2O?HSO3-+OH-���ƻ�ˮ�ĵ���ƽ�⣬��Һ�ʼ��ԣ��ʴ�Ϊ��SO32-+H2O?HSO3-+OH-��
��1��DΪKԪ�أ���Ԫ�����ڱ��е�λ���ǣ��������ڵڢ�A�壬�ʴ�Ϊ���������ڵڢ�A�壻
��2��B��D���γɵ���ͨ������ΪK2O������ʽΪ
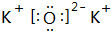 ���ʴ�Ϊ��
���ʴ�Ϊ��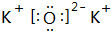 ��
����3����������ˮ��Һ�μӵ�K2S��ˮ��Һ�У���������ɫ����������������������ԭ��ͬʱ����KCl����Ӧ�Ļ�ѧ����ʽΪ��Cl2+K2S=2KCl+S�����ʴ�Ϊ��Cl2+K2S=2KCl+S����
��4��ʵ�������ȥSO2���壬����������л�ԭ�ԣ����������Ը������������ȥ�������������������������������Һ���գ�Ũ��������ն�������Ʒ����Һ���Լ����������ѡAC��
��5����A��B��D����Ԫ������ɵ�ij����ˮ��Һ�Լ��ԣ�����Ϊ�������ƣ���Һ��SO32-ˮ��SO32-+H2O?HSO3-+OH-���ƻ�ˮ�ĵ���ƽ�⣬��Һ�ʼ��ԣ��ʴ�Ϊ��SO32-+H2O?HSO3-+OH-��
���������⿼��ṹ����λ�ù�ϵӦ�ã��ѶȲ����ƶ�Ԫ���ǽ���ؼ���ע��Ի���֪ʶ���������գ�

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
�����Ŀ
A��B��C��D��ԭ������������������ֶ�����Ԫ�أ��ס��ҡ������������������е����ֻ�����Ԫ����ɵĻ������������CԪ���γɵĵ��ʣ���֪����+��=��+������+��=��+����������0.1mol/L����Һ��pHΪ13��������˵����ȷ���ǣ�������
| A��Ԫ��C�γɵĵ��ʿ����ڵ�ȼ�����ֱ���Ԫ��A��B��D�γɵĵ��ʻ��ϣ����û���������ڹ��ۼ� | B��Ԫ��B��C��D��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ��r��D����r��C����r��B�� | C��1.0 L 0.1 mol/L����Һ�к��������ܵ����ʵ���С��0.1 mol | D��1 mol��������������ȫ��Ӧ��ת��Լ1.204��1024������ |
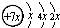 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺

 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ �����бȽ�����ȷ���ǣ�������
�����бȽ�����ȷ���ǣ�������