��Ŀ����
A��B��C��D��ԭ��������������Ķ�����Ԫ�أ������ڱ���A��B��B��C���ڣ�A��C��B��C��ɵĻ����ﶼ�ǻ������ų��Ĵ�����Ⱦ��ɷ֣�C��D���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����
��1��BԪ�ص�ԭ�ӽṹʾ��ͼΪ ��������D2C2�ĵ���ʽΪ ��
��2��A��B��C��D����Ԫ�����γɵĵ��ʾ����У��������γɷ��Ӿ����Ԫ���� ����Ԫ�ط��ű�ʾ����
��3��B���⻯���B������������Ӧˮ���ﷴӦ����Z��Z�еĻ�ѧ���������� ��
��4��AC2�Ŀռ乹���� ������ ������Է��ӡ��Ǽ��Է��ӡ�����AC2��D2C2��Ӧ�Ļ�ѧ����ʽ�� ��
��5��B��D��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��6����֪8gA�� �⻯���ʷ�ȼ�������ȶ���������ų�445.15kJ��������A���⻯��ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
��1��BԪ�ص�ԭ�ӽṹʾ��ͼΪ
��2��A��B��C��D����Ԫ�����γɵĵ��ʾ����У��������γɷ��Ӿ����Ԫ����
��3��B���⻯���B������������Ӧˮ���ﷴӦ����Z��Z�еĻ�ѧ����������
��4��AC2�Ŀռ乹����
��5��B��D��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ��
��6����֪8gA�� �⻯���ʷ�ȼ�������ȶ���������ų�445.15kJ��������A���⻯��ȼ���ȵ��Ȼ�ѧ����ʽΪ
������A��B��C��D��ԭ��������������Ķ�����Ԫ�أ�A��C��B��C��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�ΪCO��NOx�������ڱ���A��B��B��C���ڣ���AΪC��BΪN��CΪO��C��D���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ�����������ӻ�����ֱ�ΪNa2O��Na2O2����DΪNa��
��1��Nԭ�Ӻ��������Ϊ7����2�����Ӳ㣬����������ֱ�Ϊ2��5��Na2O2�������ӻ����������������������ӹ��ɣ�
��2���Ƶ������ڽ������壻
��3��������������ӻ�����������Ӽ����������笠������ж����й��ۼ���
��4��CO2��ֱ���ͽṹ������������������Ƿ��غ��жϷ��Ӽ��ԣ����������������̼��Ӧ����̼������������
��5��B��D��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּӦ��Na3N��ˮ��Ӧ��������������һˮ�ϰ���
��6������1mol����ȼ�շų���������ע�����ʾۼ�״̬���ʱ䣬ע�����Ļ�ѧ������Ϊ1���ݴ���д��
��1��Nԭ�Ӻ��������Ϊ7����2�����Ӳ㣬����������ֱ�Ϊ2��5��Na2O2�������ӻ����������������������ӹ��ɣ�
��2���Ƶ������ڽ������壻
��3��������������ӻ�����������Ӽ����������笠������ж����й��ۼ���
��4��CO2��ֱ���ͽṹ������������������Ƿ��غ��жϷ��Ӽ��ԣ����������������̼��Ӧ����̼������������
��5��B��D��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּӦ��Na3N��ˮ��Ӧ��������������һˮ�ϰ���
��6������1mol����ȼ�շų���������ע�����ʾۼ�״̬���ʱ䣬ע�����Ļ�ѧ������Ϊ1���ݴ���д��
����⣺A��B��C��D��ԭ��������������Ķ�����Ԫ�أ�A��C��B��C��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�ΪCO��NOx�������ڱ���A��B��B��C���ڣ���AΪC��BΪN��CΪO��C��D���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ�����������ӻ�����ֱ�ΪNa2O��Na2O2����DΪNa��
��1��Nԭ�Ӻ��������Ϊ7����2�����Ӳ㣬����������ֱ�Ϊ2��5����ԭ�ӽṹʾ��ͼΪ��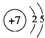 ��Na2O2�������ӻ����������������������ӹ��ɣ������ʽΪ��
��Na2O2�������ӻ����������������������ӹ��ɣ������ʽΪ��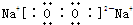 ��
��
�ʴ�Ϊ��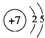 ��
��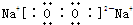 ��
��
��2��̼������C60��Ϊ���Ӿ��壬�����������ȿ����γɷ��Ӿ��壬���Ƶ������ڽ������壬
�ʴ�Ϊ��Na��
��3��������������ӻ�����������Ӽ����������笠����Ӷ�ͨ�����ۼ��γɣ�
�ʴ�Ϊ�����Ӽ������ۼ���
��4��CO2��ֱ���ͽṹ��������������������غϣ����ڷǼ��Է��ӣ����������������̼��Ӧ����̼��������������Ӧ����ʽΪ��2CO2+2Na2O2=2Na2CO3+O2��
�ʴ�Ϊ��ֱ���ͣ��Ǽ��Է��ӣ�2CO2+2Na2O2=2Na2CO3+O2��
��5��B��D��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּӦ��Na3N��ˮ��Ӧ��������������һˮ�ϰ�����Ӧ����ʽΪ��Na3N+4H2O=NH3?H2O+3NaOH��
�ʴ�Ϊ��Na3N+4H2O=NH3?H2O+3NaOH��
��6��1mol����ȼ�շų�������Ϊ445.15kJ��
=890.3kJ���ʼ���ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3kJ?mol-1��
�ʴ�Ϊ��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3kJ?mol-1��
��1��Nԭ�Ӻ��������Ϊ7����2�����Ӳ㣬����������ֱ�Ϊ2��5����ԭ�ӽṹʾ��ͼΪ��
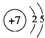 ��Na2O2�������ӻ����������������������ӹ��ɣ������ʽΪ��
��Na2O2�������ӻ����������������������ӹ��ɣ������ʽΪ��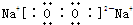 ��
���ʴ�Ϊ��
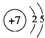 ��
��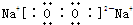 ��
����2��̼������C60��Ϊ���Ӿ��壬�����������ȿ����γɷ��Ӿ��壬���Ƶ������ڽ������壬
�ʴ�Ϊ��Na��
��3��������������ӻ�����������Ӽ����������笠����Ӷ�ͨ�����ۼ��γɣ�
�ʴ�Ϊ�����Ӽ������ۼ���
��4��CO2��ֱ���ͽṹ��������������������غϣ����ڷǼ��Է��ӣ����������������̼��Ӧ����̼��������������Ӧ����ʽΪ��2CO2+2Na2O2=2Na2CO3+O2��
�ʴ�Ϊ��ֱ���ͣ��Ǽ��Է��ӣ�2CO2+2Na2O2=2Na2CO3+O2��
��5��B��D��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּӦ��Na3N��ˮ��Ӧ��������������һˮ�ϰ�����Ӧ����ʽΪ��Na3N+4H2O=NH3?H2O+3NaOH��
�ʴ�Ϊ��Na3N+4H2O=NH3?H2O+3NaOH��
��6��1mol����ȼ�շų�������Ϊ445.15kJ��
| 1mol��16g/mol |
| 8g |
�ʴ�Ϊ��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3kJ?mol-1��
���������⿼��Ԫ�ػ������ƶϡ�����ʽ����ѧ�����Ȼ�ѧ����ʽ�����ӽṹ�����ʵȣ��Ѷ��еȣ���6��ע��ȼ���ȵ��Ȼ�ѧ����ʽ�п�ȼ��Ļ�ѧ������Ϊ1���������ȶ��������

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ
A��B��C��D��ԭ������������������ֶ�����Ԫ�أ��ס��ҡ������������������е����ֻ�����Ԫ����ɵĻ������������CԪ���γɵĵ��ʣ���֪����+��=��+������+��=��+����������0.1mol/L����Һ��pHΪ13��������˵����ȷ���ǣ�������
| A��Ԫ��C�γɵĵ��ʿ����ڵ�ȼ�����ֱ���Ԫ��A��B��D�γɵĵ��ʻ��ϣ����û���������ڹ��ۼ� | B��Ԫ��B��C��D��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ��r��D����r��C����r��B�� | C��1.0 L 0.1 mol/L����Һ�к��������ܵ����ʵ���С��0.1 mol | D��1 mol��������������ȫ��Ӧ��ת��Լ1.204��1024������ |
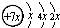 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺

 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ �����бȽ�����ȷ���ǣ�������
�����бȽ�����ȷ���ǣ�������