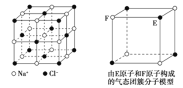
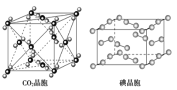
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΒΣΦΑΤδΜ·ΚœΈο‘Ύ…ζ≤ζ…ζΜν÷–”–ΙψΖΚ”Π”ΟΘ°
![]() “―÷ΣΘΚCOΩ…ΫΪ≤ΩΖ÷ΒΣΒΡ―θΜ·ΈοΜΙ‘≠ΈΣ
“―÷ΣΘΚCOΩ…ΫΪ≤ΩΖ÷ΒΣΒΡ―θΜ·ΈοΜΙ‘≠ΈΣ![]() Θ°
Θ°
Ζ¥”ΠΔώΘΚ![]()
Ζ¥”ΠΔρΘΚ![]()
–¥≥ωCOΫΪ![]() ΜΙ‘≠ΈΣNOΒΡ»»Μ·―ßΖΫ≥Χ Ϋ ______ Θ°
ΜΙ‘≠ΈΣNOΒΡ»»Μ·―ßΖΫ≥Χ Ϋ ______ Θ°
![]() ‘ΎΟή±’»ίΤς÷–≥δ»κ5molCOΚΆ4molNOΘ§ΖΔ…ζ…œ ωΖ¥”ΠIΘ§ΆΦ1ΈΣΤΫΚβ ±NOΒΡΧεΜΐΖ÷ ΐ”κΈ¬Ε»ΓΔ―Ι«ΩΒΡΙΊœΒΘ°
‘ΎΟή±’»ίΤς÷–≥δ»κ5molCOΚΆ4molNOΘ§ΖΔ…ζ…œ ωΖ¥”ΠIΘ§ΆΦ1ΈΣΤΫΚβ ±NOΒΡΧεΜΐΖ÷ ΐ”κΈ¬Ε»ΓΔ―Ι«ΩΒΡΙΊœΒΘ°
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
![]() Έ¬Ε»ΘΚ
Έ¬Ε»ΘΚ![]() ______
______ ![]() ΧνΓΑ
ΧνΓΑ![]() Γ±ΜρΓΑ
Γ±ΜρΓΑ![]() Γ±
Γ±![]() Θ°
Θ°
![]() Ρ≥Έ¬Ε»œ¬Θ§‘ΎΧεΜΐΈΣ2LΒΡΟή±’»ίΤς÷–Θ§Ζ¥”ΠΫχ––10Ζ÷÷”Ζ≈≥ω»»ΝΩ373kJΘ§”ΟCOΒΡ≈®Ε»±δΜ·±μ ΨΒΡΤΫΨυΖ¥”ΠΥΌ¬
Ρ≥Έ¬Ε»œ¬Θ§‘ΎΧεΜΐΈΣ2LΒΡΟή±’»ίΤς÷–Θ§Ζ¥”ΠΫχ––10Ζ÷÷”Ζ≈≥ω»»ΝΩ373kJΘ§”ΟCOΒΡ≈®Ε»±δΜ·±μ ΨΒΡΤΫΨυΖ¥”ΠΥΌ¬ ![]() ______ Θ°
______ Θ°
![]() Ρ≥Έ¬Ε»œ¬Θ§Ζ¥”Π¥οΒΫΤΫΚβΉ¥Χ§DΒψ ±Θ§»ίΤςΧεΜΐΈΣ2LΘ§¥Υ ±ΒΡΤΫΚβ≥Θ ΐ
Ρ≥Έ¬Ε»œ¬Θ§Ζ¥”Π¥οΒΫΤΫΚβΉ¥Χ§DΒψ ±Θ§»ίΤςΧεΜΐΈΣ2LΘ§¥Υ ±ΒΡΤΫΚβ≥Θ ΐ![]() ______
______ ![]() ΫαΙϊΨΪ»ΖΒΫ
ΫαΙϊΨΪ»ΖΒΫ![]() ΘΜ»τ‘ΎDΒψΕ‘Ζ¥”Π»ίΤς…ΐΈ¬ΒΡΆ§ ±ά©¥σΧεΜΐ ΙΧεœΒ―Ι«ΩΦθ–ΓΘ§÷Ί–¬¥οΒΫΒΡΤΫΚβΉ¥Χ§Ω…Ρή «ΆΦ÷–
ΘΜ»τ‘ΎDΒψΕ‘Ζ¥”Π»ίΤς…ΐΈ¬ΒΡΆ§ ±ά©¥σΧεΜΐ ΙΧεœΒ―Ι«ΩΦθ–ΓΘ§÷Ί–¬¥οΒΫΒΡΤΫΚβΉ¥Χ§Ω…Ρή «ΆΦ÷–![]() Βψ÷–ΒΡ ______ Βψ
Βψ÷–ΒΡ ______ Βψ
![]() Ρ≥Έ¬Ε» ±Θ§―«œθΥα“χ
Ρ≥Έ¬Ε» ±Θ§―«œθΥα“χ![]() ΒΡ
ΒΡ![]() ΓΔ
ΓΔ![]() ΒΡ
ΒΡ![]() Θ§Β±œρΚ§
Θ§Β±œρΚ§![]() ΓΔ
ΓΔ![]() ΜλΚœ»ή“Κ÷–Φ”»κ
ΜλΚœ»ή“Κ÷–Φ”»κ![]() »ή“Κ÷Ν
»ή“Κ÷Ν![]() «ΓΚΟΆξ»Ϊ≥ΝΒμ
«ΓΚΟΆξ»Ϊ≥ΝΒμ![]() Φ¥
Φ¥![]() ≈®Ε»Β»”Ύ
≈®Ε»Β»”Ύ![]()
![]() ±Θ§
±Θ§![]() ______ Θ°
______ Θ°
![]() »γΆΦ2Θ§‘ΎΥα–‘ΧθΦΰœ¬Θ§ΒγΫβΥ°÷–
»γΆΦ2Θ§‘ΎΥα–‘ΧθΦΰœ¬Θ§ΒγΫβΥ°÷–![]() Ω…ΉΣΜ·ΈΣ
Ω…ΉΣΜ·ΈΣ![]() ΚΆ
ΚΆ![]() Θ§«κ–¥≥ω―τΦΪΒΡΒγΦΪΖ¥”Π Ϋ ______ Θ°
Θ§«κ–¥≥ω―τΦΪΒΡΒγΦΪΖ¥”Π Ϋ ______ Θ°
ΓΨ¥πΑΗΓΩ![]()
![]()
![]()
![]() A
A ![]()
![]()
ΓΨΫβΈωΓΩ
Θ®1Θ©Ζ¥”ΠΔώΘΚ![]()
Ζ¥”ΠΔρΘΚ![]()
ΗυΨίΗ«ΥΙΕ®¬…Θ§![]() ΒΟΒΫ
ΒΟΒΫ![]() Θ§Ψί¥ΥΦΤΥψΘΜ
Θ§Ψί¥ΥΦΤΥψΘΜ
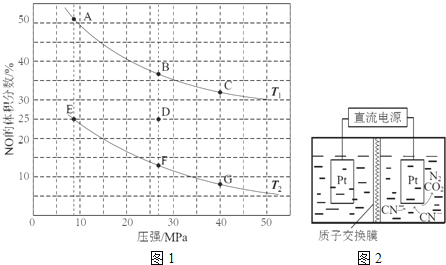
Θ®2Θ©ΔΌΗυΨίΖ¥”Π![]() Θ§…ΐΗΏΈ¬Ε»Θ§ΤΫΚβΡφœρ“ΤΕ·Θ§Υυ“‘NOΒΡΧεΜΐΖ÷ ΐΜα‘ω¥σΘΜ
Θ§…ΐΗΏΈ¬Ε»Θ§ΤΫΚβΡφœρ“ΤΕ·Θ§Υυ“‘NOΒΡΧεΜΐΖ÷ ΐΜα‘ω¥σΘΜ
ΔΎ”ΟCOΒΡ≈®Ε»±δΜ·±μ ΨΒΡΤΫΨυΖ¥”ΠΥΌ¬ ![]() ΫαΚœ»»Μ·―ßΖΫ≥Χ ΫΒΡ“β“εΜΊ¥πΦΤΥψΘΜ
ΫαΚœ»»Μ·―ßΖΫ≥Χ ΫΒΡ“β“εΜΊ¥πΦΤΥψΘΜ
ΔέΗυΨί»ΐ–– ΫΫαΚœΜ·―ßΤΫΚβ“ΤΕ·‘≠άμά¥ΜΊ¥πΘΜ
Θ®3Θ©ΗυΨί≥ΝΒμ»ήΫβΤΫΚβ≥Θ ΐKspΫχ––ΦΤΥψΘΜ
Θ®4Θ©‘ΎΒγΫβ≥ΊΒΡ―τΦΪ…œΖΔ…ζ ßΒγΉ”ΒΡ―θΜ·Ζ¥”ΠΘ§Ψί¥Υ ι–¥ΒγΦΪΖ¥”ΠΘ°
ΫβΘΚ![]() “―÷ΣΘΚΖ¥”ΠΔώΘΚ
“―÷ΣΘΚΖ¥”ΠΔώΘΚ![]()
Ζ¥”ΠΔρΘΚ![]()
ΗυΨίΗ«ΥΙΕ®¬…Θ§![]() Δρ
Δρ![]() Δώ
Δώ![]() ΒΟΒΫ
ΒΟΒΫ![]() Θ§
Θ§![]() ΘΜ
ΘΜ
Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
![]() ΗυΨίΖ¥”Π
ΗυΨίΖ¥”Π![]() Θ§…ΐΗΏΈ¬Ε»Θ§ΤΫΚβΡφœρ“ΤΕ·Θ§Υυ“‘NOΒΡΧεΜΐΖ÷ ΐΜα‘ω¥σΘ§Φ¥
Θ§…ΐΗΏΈ¬Ε»Θ§ΤΫΚβΡφœρ“ΤΕ·Θ§Υυ“‘NOΒΡΧεΜΐΖ÷ ΐΜα‘ω¥σΘ§Φ¥![]() Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
![]() ‘ΎΧεΜΐΈΣ2LΒΡΟή±’»ίΤς÷–Θ§Ζ¥”ΠΫχ––10Ζ÷÷”Ζ≈≥ω»»ΝΩ373kJΘ§ΗυΨίΖ¥”ΠΘΚ
‘ΎΧεΜΐΈΣ2LΒΡΟή±’»ίΤς÷–Θ§Ζ¥”ΠΫχ––10Ζ÷÷”Ζ≈≥ω»»ΝΩ373kJΘ§ΗυΨίΖ¥”ΠΘΚ![]() Θ§œϊΚΡCOΒΡΈο÷ ΒΡΝΩ «
Θ§œϊΚΡCOΒΡΈο÷ ΒΡΝΩ «![]() Θ§
Θ§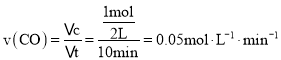 ΘΜΙ ¥πΑΗΈΣΘΚ
ΘΜΙ ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
![]() Ρ≥Έ¬Ε»œ¬Θ§Ζ¥”Π¥οΒΫΤΫΚβΉ¥Χ§DΒψ ±Θ§NOΒΡΧεΜΐΖ÷ ΐ «
Ρ≥Έ¬Ε»œ¬Θ§Ζ¥”Π¥οΒΫΤΫΚβΉ¥Χ§DΒψ ±Θ§NOΒΡΧεΜΐΖ÷ ΐ «![]() Θ§…ηCOΒΡ±δΜ·≈®Ε» «xΘ§
Θ§…ηCOΒΡ±δΜ·≈®Ε» «xΘ§
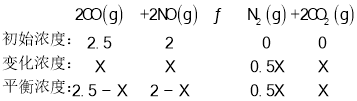
NOΒΡΧεΜΐΖ÷ ΐ «![]() Θ§Φ¥
Θ§Φ¥![]() Θ§ΫβΒΟ
Θ§ΫβΒΟ![]() Θ§¥Υ ±
Θ§¥Υ ±![]() ΘΜ»τ‘ΎDΒψΕ‘Ζ¥”Π»ίΤς…ΐΈ¬ΒΡΆ§ ±ά©¥σΧεΜΐ ΙΧεœΒ―Ι«ΩΦθ–ΓΘ§‘ρΤΫΚβΜαΡφœρ“ΤΕ·Θ§NOΒΡΧεΜΐΖ÷ ΐ‘ωΦ”Θ§÷Ί–¬¥οΒΫΒΡΤΫΚβΉ¥Χ§Ω…Ρή «ΆΦ÷–AΒψΘ§
ΘΜ»τ‘ΎDΒψΕ‘Ζ¥”Π»ίΤς…ΐΈ¬ΒΡΆ§ ±ά©¥σΧεΜΐ ΙΧεœΒ―Ι«ΩΦθ–ΓΘ§‘ρΤΫΚβΜαΡφœρ“ΤΕ·Θ§NOΒΡΧεΜΐΖ÷ ΐ‘ωΦ”Θ§÷Ί–¬¥οΒΫΒΡΤΫΚβΉ¥Χ§Ω…Ρή «ΆΦ÷–AΒψΘ§
Ι ¥πΑΗΈΣΘΚ![]() ΘΜAΘΜ
ΘΜAΘΜ
![]() Β±œρΚ§
Β±œρΚ§![]() ΓΔ
ΓΔ![]() ΜλΚœ»ή“Κ÷–Φ”»κ
ΜλΚœ»ή“Κ÷–Φ”»κ![]() »ή“Κ÷Ν
»ή“Κ÷Ν![]() «ΓΚΟΆξ»Ϊ≥ΝΒμΘ§ΗυΨί
«ΓΚΟΆξ»Ϊ≥ΝΒμΘ§ΗυΨί![]() ΒΡ
ΒΡ![]() Θ§¥Υ ±“χάκΉ”≈®Ε»
Θ§¥Υ ±“χάκΉ”≈®Ε»![]() Θ§
Θ§![]()
![]() ΘΜΙ ¥πΑΗΈΣΘΚ
ΘΜΙ ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
![]() ‘ΎΥα–‘ΧθΦΰœ¬Θ§ΒγΫβΥ°÷–
‘ΎΥα–‘ΧθΦΰœ¬Θ§ΒγΫβΥ°÷–![]() Ω…ΉΣΜ·ΈΣ
Ω…ΉΣΜ·ΈΣ![]() ΚΆ
ΚΆ![]() Θ§―τΦΪ…œΖΔ…ζ ßΒγΉ”ΒΡ―θΜ·Ζ¥”ΠΘ§ΤδΒγΦΪΖ¥”Π ΫΈΣΘΚ
Θ§―τΦΪ…œΖΔ…ζ ßΒγΉ”ΒΡ―θΜ·Ζ¥”ΠΘ§ΤδΒγΦΪΖ¥”Π ΫΈΣΘΚ![]() Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ![]() ΓΘ
ΓΘ

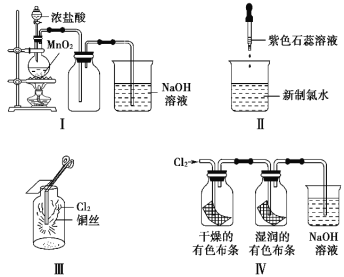
ΓΨΧβΡΩΓΩΥ° «…ζΟϋΒΡ‘¥»ΣΓΔΙΛ“ΒΒΡ―Σ“ΚΓΔ≥« –ΒΡΟϋ¬ωΓΘ“Σ±ΘΜΛΚΟΚ”ΝςΘ§Κ”Υ° «÷Ί“ΣΒΡ“ϊ”ΟΥ°‘¥Θ§Έέ»ΨΈοΆ®Ιΐ“ϊ”ΟΥ°Ω…÷±Ϋ”ΕΨΚΠ»ΥΧεΓΘ“≤Ω…Ά®Ιΐ ≥ΈοΝ¥ΚΆΙύΗ»≈©ΧοΦδΫ”ΈΘΦΑΫΓΩΒΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)25Γφ ±Θ§œρΥ°ΒΡΒγάκΤΫΚβΧεœΒ÷–Φ”»κ…ΌΝΩΧΦΥαΡΤΙΧΧεΘ§ΒΟΒΫpHΈΣ11ΒΡ»ή“ΚΘ§ΤδΥ°ΫβΒΡάκΉ”ΖΫ≥Χ ΫΈΣ________Θ§”…Υ°Βγάκ≥ωΒΡc(OH-)=_______mol/L
(2)¥ΩΥ°‘Ύ100Γφ ±Θ§pH=6Θ§ΗΟΈ¬Ε»œ¬1mol/LΒΡNaOH»ή“Κ÷–Θ§”…Υ°Βγάκ≥ωΒΡ c(OH-)=_______mol/LΓΘ
(3)ΧεΜΐΨυΈΣ100mLΓΔpHΨυΈΣ2ΒΡCH3COOH»ή“Κ”κ“Μ‘ΣΥαHX»ή“ΚΘ§Φ”Υ°œΓ ΆΙΐ≥Χ÷–pH”κ»ή“ΚΧεΜΐΒΡΙΊœΒ»γœ¬ΆΦΥυ ΨΘ§‘ρHXΒΡΒγάκΤΫΚβ≥Θ ΐ___(ΧνΓ±¥σ”ΎΓ±Γ±–Γ”ΎΓ±ΜρΓΑΒ»”ΎΓ±)CH3COOHΒΡΒγάκΤΫΚβ≥Θ ΐΓΘ
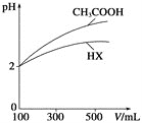
(4)ΒγάκΤΫΚβ≥Θ ΐ «ΚβΝΩ»θΒγΫβ÷ Βγάκ≥ΧΕ»«Ω»θΒΡΈοάμΝΩΓΘ“―÷ΣΘΚ
Μ·―ß Ϋ | ΒγάκΤΫΚβ≥Θ ΐ(25Γφ) |
HCN | K=4.9ΓΝ10-10 |
CH3COOH | K==1.8ΓΝ10-5 |
H2CO3 | K1=4.3ΓΝ10-7ΓΔK2=5.610-11 |
ΔΌ25Γφ ±Θ§”–Β»≈®Ε»ΒΡNaCN»ή“ΚΓΔNa2CO3“ΚΚΆCH3COONa»ή“ΚΘ§»ΐ÷÷»ή“ΚΒΡpH”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ___ΓΘ
ΔΎœρNaCN»ή“Κ÷–Ά®»κ…ΌΝΩΒΡCO2Θ§ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______
(5)25Γφ ±Θ§‘ΎCH3COOH”κCH3COONaΒΡΜλΚœ»ή“Κ÷–Θ§»τ≤βΒΟpH=6Θ§‘ρ»ή“Κ÷– c(CH3COO-)-c(Na+)=_____(ΧνΨΪ»Ζ÷Β)mol/L
ΓΨΧβΡΩΓΩœ¬Ν– Β―ι≤ΌΉς’ΐ»Ζ«“Ρή¥οΒΫœύ”ΠΡΩΒΡΒΡ «
―Γœν | Β―ιΡΩΒΡ | Β―ι≤ΌΉς |
A | ≥Τ»Γ2.0gNaOHΙΧΧε | œ»‘ΎΆ–≈Χ…œΗςΖ≈1’≈¬Υ÷ΫΘ§»ΜΚσ‘Ύ”“≈Χ…œΧμΦ”2gμά¬κΘ§Ήσ≈Χ…œΧμΦ”NaOHΙΧΧε |
B | ≈δ÷ΤœΓΝρΥα | œ»ΫΪ≈®ΝρΥαΦ”»κ…’±≠Θ§ΚσΒΙ»κ’τΝσΥ° |
C | ―ι÷ΛΧζΒΡΈϋ―θΗ· ¥ | ΫΪΧζΕΛΖ≈»κ ‘Ιή÷–Θ§”Ο―ΈΥαΫΰΟΜ |
D | Φλ―ι»ή“Κ÷– «Ζώ”–NH4+ | »Γ…ΌΝΩ ‘“Κ”Ύ ‘Ιή÷–Θ§Φ”»κNaOH»ή“Κ≤ΔΦ”»»Θ§”Ο Σ»σΒΡΚλ…Ϊ ·»ο ‘÷ΫΦλ―ι≤ζ…ζΒΡΤχΧε |
A. A B. B C. C D. D
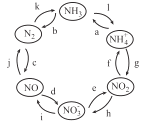
ΓΨΧβΡΩΓΩΦΉΓΔ““ΓΔ±ϊΓΔΕΓΨυΈΣ÷–―ßΜ·―ß÷–≥ΘΦϊΒΡΒΞ÷ ΜρΜ·ΚœΈοΘ§ΥϋΟ«÷°ΦδΒΡΉΣΜ·ΙΊœΒ»γœ¬ΆΦΥυ Ψ(≤ΩΖ÷≤ζΈο“―¬‘»Ξ)Θ§œ¬Ν–ΗςΉιΈο÷ ÷–≤ΜΡήΑ¥ΆΦ ΨΙΊœΒΉΣΜ·ΒΡ «
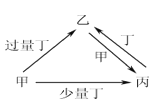
―Γœν | ΦΉ | ““ | ±ϊ | ΕΓ |
A | NaOH | NaHSO3 | Na2SO3 | SO2 |
B | Fe | Fe(NO3)3 | Fe(NO3)2 | HNO3 |
C | C | CO2 | CO | O2 |
D | Al | NaAlO2 | Al(OH)3 | NaOH |
A. A B. B C. C D. D