��Ŀ����
17�������£�������5����Һ| �� | �� | �� | �� | �� |
| 0.1mol•L-1CH3COOH��Һ | 0.01mol•L-1CH3COOH��Һ | pH=3��CH3COOH��Һ | 0.1mol•L-1NaOH��Һ | 0.1mol•L-1��ˮ |
��1����Һ��ϡ�͵�ԭ����100�������ҺpH������Һ��pH���������=����������ͬ�����ٺ͢�����Һ��ˮ�������c��H+�����٣��ܣ�
��2������ͬ�¶�ʱ��10mL �ٵ���Һ��100mL �ڵ���Һ��Ƚϣ�������ֵ���ߴ���ǰ�ߵ���BC������ĸ����
A���к�ʱ����NaOH���� B������̶� C��ˮ�������c��H+�� D��CH3COOH�����ʵ���
��3����ˮϡ�� ��ʱ����Һ������ˮ�������Ӷ���С����AB������ĸ����
A��C��OH-��B��$\frac{c��N{H}_{3}•{H}_{2}O��}{c��O{H}^{-}��}$C��C��H+����C��OH-���ij˻� D��OH-�����ʵ���
��4���������ȡ�pH��ȵ� ����Һ��������Һ�м�������Zn������Һ�в���������������в���������ࣨ��ࡱ�����١�����ȡ�����
���� ��1�����������ᣬ������ȫ���룬0.1mol•L-1CH3COOH��Һϡ��100����Ũ��Ϊ0.0001mol/L��0.1mol•L-1CH2COOH��Һ��c��H+��С��0.1mol/L��0.1mol•L-1NaOH��Һ��c��OH-��=0.1mol/L���ݴ˷�����
��2������ͬ�¶�ʱ��10mL 0.1mol•L-1CH3COOH��Һ��100mL 0.01mol•L-1CH2COOH��Һ������������ʵ�����ͬ������Ũ��ԽС�������̶�Խ��������Ũ�ȼ�С���ݴ˷�����
��3����ˮϡ��0.1mol/L��ˮʱ����Һ������ˮ�������ӣ���NH3��H2O?OH-+NH4+��֪��n��OH-��������Һ���������Ķ࣬��c��OH-����С����ˮ�ٽ����룬��n��NH3��H2O�����٣�
��4�����������ᣬû����ȫ���룬������ǿ�ᣬ�Ѿ���ȫ���룮
��� �⣺��1�����������ᣬ������ȫ���룬0.0001mol/L��pH����4��0.1mol•L-1CH2COOH��Һ��c��H+��С��0.1mol/L��0.1mol•L-1NaOH��Һ��c��OH-��=0.1mol/L��c��H+����c��OH-��Խ�����ˮ�ĵ���ƽ���Ӱ��Խ��������ˮ���������������Ũ�Ȣ٣��ܣ��ʴ�Ϊ����������
��2��10mL 0.1mol•L-1CH3COOH��Һ��100mL 0.01mol•L-1CH2COOH��Һ������������ʵ�����ͬ�������к�NaOH�����ʵ�����ͬ����AD����
����Ũ��ԽС�������̶�Խ����Ũ��ԽС��������Խ����������Ũ�ȼ�С����ˮ�ĵ���ƽ���Ӱ��ԽС����BC��ȷ��
�ʴ�Ϊ��BC��
��3��A����ˮ��ˮϡ�ͣ���Һ��Ũ�ȼ�С�����Լ�������������Ũ�ȼ�С�������¶Ȳ��䣬��c��H+����c��OH-���ij˻����䣬������������Ũ�ȼ�С��������Ũ������A��ȷ��
B����NH3��H2O?OH-+NH4+��֪����ˮ�ٽ����룬��c��NH3��H2O�����٣�c��OH-����С������ƽ�������ƶ�����ˮ��Ũ�ȼ�С�ö࣬���Է�ĸ��С�ij̶ȴ��Ӽ�С�ij̶�С�����ߵı�ֵ��С����B��ȷ��
C�����ˮϡ��ʱ���¶Ȳ��䣬��c��H+����c��OH-���ij˻����䣬��C����
D����NH3��H2O?OH-+NH4+��֪����ˮ�ٽ����룬OH-�����ʵ�������D����
�ʴ�Ϊ��AB��
��4�����ڴ��Ჿ�ֵ��룬������ȫ���룬����pH��ȡ������ȵĴ����������Һ�м�������Zn����������������࣬�ʴ�Ϊ���࣮
���� ���⿼���������ˮϡ��ʱpH�仯��Ӱ��ˮ�ĵ���ƽ���ƶ������ء�Ӱ��������ʵ���ƽ���ƶ������صȣ�ע�����Ჿ�ֵ��룬��Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�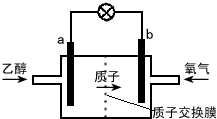
| A�� | a��Ϊ��صĸ������õ缫����������Ӧ | |
| B�� | ��ع���ʱ������a�������ص��߾����ݵ�b�� | |
| C�� | ��������ĵ缫��ӦʽΪ O2+2H2O+4e-�T4OH- | |
| D�� | ��ع���ʱ��1mol�Ҵ�������ת��12mol���� |
| A�� | v ��NH3��=0.2 mol/��L•s�� | B�� | v ��O2��=1.4mol/��L•min�� | ||
| C�� | v ��H2O��=0.25 mol/��L•s�� | D�� | v ��NO��=0.9 mol/��L•min�� |
| ��������ĩ״��/mol | ���Ũ�ȼ���� | ��Ӧ�¶� | |||
| A | Mg | 0.1 | 3mol•L-1���� | 10mL | 25�� |
| B | Fe | 0.1 | 3mol•L-1���� | 10mL | 25�� |
| C | Mg | 0.1 | 3mol•L-1���� | 10mL | 25�� |
| D | Mg | 0.1 | 6mol•L-1���� | 10mL | 60�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��������CH3COONa���壬ƽ��������Ӧ�����ƶ� | |
| B�� | ��������NaOH���壬ƽ��������Ӧ�����ƶ� | |
| C�� | ͨ������HCl���壬��Һ��pHֵ���� | |
| D�� | ����ˮʱ��ƽ�����淴Ӧ�����ƶ� |
| A�� | NaOH | B�� | H2O | C�� | CaCl2 | D�� | H2SO4 |
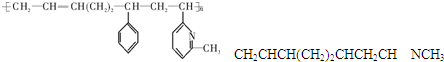
�䵥�����������6�ֵļ��֣�
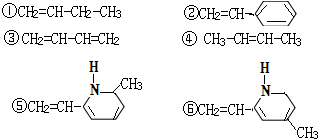
��CH2CHNCH3
��ȷ������ǣ�������
| A�� | �ڢۢ� | B�� | �ڢۢ� | C�� | �٢ڢ� | D�� | �ڢܢ� |
| ���� | CH4 | CH3-CH3 | CH2�TCH2 |
| ���ʵ������� | 20% | 30% | 50% |
��2�������֣�ʯ�������Ƭ��Ϲ��ȵIJ����У�����̬��CH4��C2H6��CH2�TCH2�⣬������һ��Һ̬�����ɣ�0.2mol��Һ̬����O2����ȫȼ��ʱ���õ�����������������ʵ�����Ϊ1.2mol��������ķ���ʽΪC6H12��������ͬ���칹���ж��֣���������̼ԭ�Ӿ�����ͬһƽ��Ľṹ��ʽΪ
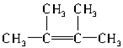 ��
��