��Ŀ����
��1��д���������ʵĵ��뷽��ʽ��
NaHCO3
Al2��SO4��3
��2��д�����з�Ӧ�����ӷ���ʽ��
Fe2O3��ϡ���
ϡ����������������Ӧ��
��3�������з�Ӧ�У�
A.2F2+2H2O�T4HF+O2 B.2Na+2H2O�T2NaOH+H2��
C��CaO+H2O�TCa��OH��2 D.2H2O�T2H2��+O2��
ˮֻ������������
NaHCO3
NaHCO3�TNa++HCO3-
NaHCO3�TNa++HCO3-
��Al2��SO4��3
Al2��SO4��3�T2Al3++3SO42-
Al2��SO4��3�T2Al3++3SO42-
����2��д�����з�Ӧ�����ӷ���ʽ��
Fe2O3��ϡ���
Fe2O3+6H+�T2Fe3++3H2O
Fe2O3+6H+�T2Fe3++3H2O
��ϡ����������������Ӧ��
Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O
Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O
��3�������з�Ӧ�У�
A.2F2+2H2O�T4HF+O2 B.2Na+2H2O�T2NaOH+H2��
C��CaO+H2O�TCa��OH��2 D.2H2O�T2H2��+O2��
ˮֻ������������
B
B
��ˮֻ����ԭ������A
A
����������1��̼����������ǿ����ʣ���ȫ��������̼��������Ӻ������ӣ���������ǿ����ʣ�����������Ӻ���������ӣ�
��2����������ϡ���ᷴӦ�������Ȼ�����ˮ��ϡ����������������Ӧ�������ᱵ������ˮ��
��3�����ݷ�Ӧ����ʽ�л��ϼ۱仯�ж�ˮ�ڷ�Ӧ�е����ã�
��2����������ϡ���ᷴӦ�������Ȼ�����ˮ��ϡ����������������Ӧ�������ᱵ������ˮ��
��3�����ݷ�Ӧ����ʽ�л��ϼ۱仯�ж�ˮ�ڷ�Ӧ�е����ã�
����⣺��1��NaHCO3Ϊǿ�������ȫ���룬���뷽��ʽΪ��NaHCO3�TNa++HCO3-����������ǿ����ʣ�����ȫ���룬���뷽��ʽΪ��Al2��SO4��3�T2Al3++3SO42-��
�ʴ�Ϊ��NaHCO3�TNa++HCO3-��Al2��SO4��3�T2Al3++3SO42-��
��2���������ǽ�����������ڼ���������ܺ����ᷴӦ�����Ȼ�����ˮ����Ӧ�����ӷ���ʽΪ��Fe2O3+6H+�T2Fe3++3H2O����������Ϊǿ�����Ϊǿ�ᣬ���ɲ�֣����ɵ����ᱵΪ�����ˮΪ����������ɲ�֣����ӷ���ʽΪ��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��
�ʴ�Ϊ��Fe2O3+6H+�T2Fe3++3H2O��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��
��3��A.2F2+2H2O�T4HF+O2����Ӧ��ˮ�е���Ԫ�ر�����������������ˮֻ����ԭ����
B.2Na+2H2O�T2NaOH+H2����ˮ�е���Ԫ�ر���ԭ������������ˮֻ����������
C��CaO+H2O�TCa��OH��2���÷�Ӧ������������ԭ��Ӧ��ˮ�Ȳ���������Ҳ���ǻ�ԭ����
D.2H2O�T2H2��+O2�������ˮ��ˮ����������Ҳ�ǻ�ԭ����
�������Ϸ�����ˮֻ�����������ǣ�B��ˮֻ����ԭ�����ǣ�A��
�ʴ�Ϊ��B��A��
�ʴ�Ϊ��NaHCO3�TNa++HCO3-��Al2��SO4��3�T2Al3++3SO42-��
��2���������ǽ�����������ڼ���������ܺ����ᷴӦ�����Ȼ�����ˮ����Ӧ�����ӷ���ʽΪ��Fe2O3+6H+�T2Fe3++3H2O����������Ϊǿ�����Ϊǿ�ᣬ���ɲ�֣����ɵ����ᱵΪ�����ˮΪ����������ɲ�֣����ӷ���ʽΪ��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��
�ʴ�Ϊ��Fe2O3+6H+�T2Fe3++3H2O��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��
��3��A.2F2+2H2O�T4HF+O2����Ӧ��ˮ�е���Ԫ�ر�����������������ˮֻ����ԭ����
B.2Na+2H2O�T2NaOH+H2����ˮ�е���Ԫ�ر���ԭ������������ˮֻ����������
C��CaO+H2O�TCa��OH��2���÷�Ӧ������������ԭ��Ӧ��ˮ�Ȳ���������Ҳ���ǻ�ԭ����
D.2H2O�T2H2��+O2�������ˮ��ˮ����������Ҳ�ǻ�ԭ����
�������Ϸ�����ˮֻ�����������ǣ�B��ˮֻ����ԭ�����ǣ�A��
�ʴ�Ϊ��B��A��
���������⿼��ѧ�����ӷ���ʽ����д֪ʶ����ԭ���ͻ�ԭ�����жϣ����Ը�����ѧ֪ʶ���лش������ڿ��Ե��ȵ㣬�ѶȲ�����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
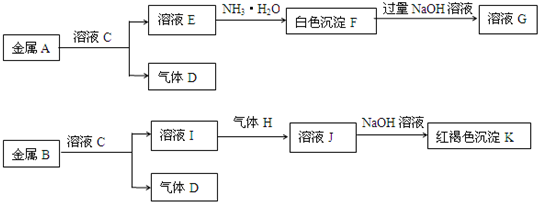
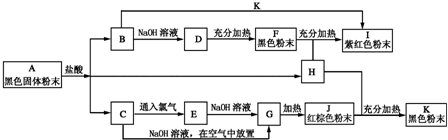
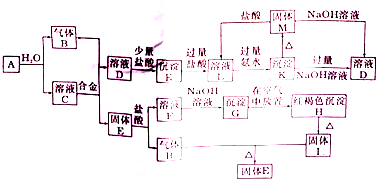
 ��ͼ���������ʵ��ת����ϵ����֪E�ǹ�̬���ʣ������ڻ�ɽ�ڴ������������о����й���E��Ԫ�أ�
��ͼ���������ʵ��ת����ϵ����֪E�ǹ�̬���ʣ������ڻ�ɽ�ڴ������������о����й���E��Ԫ�أ�