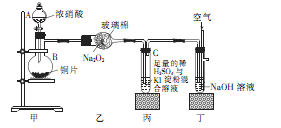
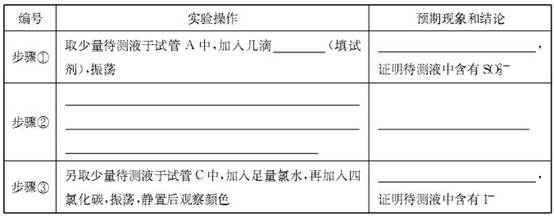
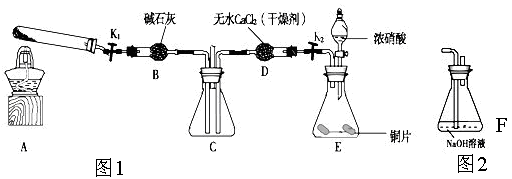
��Ŀ����
��֪A������ͼ��ʾ�Ĺ���ת��ΪD�� 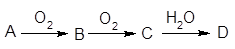
��ش��������⣺
��1����AΪ�ǽ������ʣ��ҳ�����Ϊ����ɫ���壬BΪ�̼�����ζ����ɫ���壬��ʹƷ����Һ��ɫ��DΪǿ�ᡣ
��D�Ļ�ѧʽ�� ��
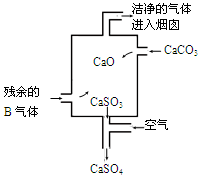
���ڹ�ҵ������B����Ĵ����ŷű���ˮ���պ��γ��� ����Ⱦ�˻�����ij�������������B����װ����ͼ�����ȥB������ܷ�Ӧ����ʽ�� ��
��2����A����ʹ��̪��Һ�������塣D��һ��ǿ�ᡣд��A��B�Ļ�ѧ����ʽ ����ҵ�����ϣ���34��AΪԭ�ϣ�����������63%��D�� �֡�
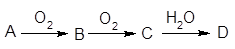
��ش��������⣺
��1����AΪ�ǽ������ʣ��ҳ�����Ϊ����ɫ���壬BΪ�̼�����ζ����ɫ���壬��ʹƷ����Һ��ɫ��DΪǿ�ᡣ
��D�Ļ�ѧʽ�� ��
���ڹ�ҵ������B����Ĵ����ŷű���ˮ���պ��γ��� ����Ⱦ�˻�����ij�������������B����װ����ͼ�����ȥB������ܷ�Ӧ����ʽ�� ��

��2����A����ʹ��̪��Һ�������塣D��һ��ǿ�ᡣд��A��B�Ļ�ѧ����ʽ ����ҵ�����ϣ���34��AΪԭ�ϣ�����������63%��D�� �֡�
��1����H2SO4��2�֣������꣨1�֣���2SO2 +2CaCO3+O2�� 2CaSO4+2CO2��2�֣�
(2) 4NH3��5O2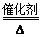 4NO��6H2O ��2�֣���200��2�֣�
4NO��6H2O ��2�֣���200��2�֣�
(2) 4NH3��5O2
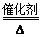 4NO��6H2O ��2�֣���200��2�֣�
4NO��6H2O ��2�֣���200��2�֣������������1����AΪ�ǽ������ʣ��ҳ�����Ϊ����ɫ���壬��A�ǵ���S������������ȼ������SO2��SO2�Ǵ̼�����ζ����ɫ���壬��B��SO2��SO2��ʹƷ����Һ��ɫ��SO2�����õ���������������������ˮ�������ᣬ��DΪǿ�����ᡣ
��D�Ļ�ѧʽ��H2SO4��
���ڹ�ҵ������SO2����Ĵ����ŷű���ˮ���պ��γ����������Ⱦ�˻���������ʾ��ͼ��֪��̼��Ƹ��·ֽ����������ƺ�CO2������������SO2����������ơ�������Ʋ��ȶ����������е�����������������ƣ��Ӷ���ֹ������Ⱦ����˷�Ӧ���ܻ�ѧ����ʽΪ2SO2 +2CaCO3+O2��2CaSO4+2CO2��
��2����A����ʹ��̪��Һ�������壬��A�ǰ����������������з�������������NO��ˮ��NO��������������NO2��NO2����ˮ����ǿ�����ᣬ���A��B�Ļ�ѧ����ʽΪ4NH3��5O2
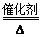 4NO��6H2O�����ݵ�ԭ���غ��֪��
4NO��6H2O�����ݵ�ԭ���غ��֪��NH3��������HNO3
17t 63t
34t m��63%
���m��
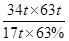 ��200t
��200t
��ϰ��ϵ�д�
�����Ŀ