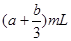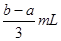��Ŀ����
��������(N2H4)�ǵ������ֳ���������ڿ�ѧ����������������Ҫ��Ӧ�á�����
����������м��㣺
�������������������ɵ�����һ���⻯����⻯�����Է�������Ϊ43.0�����е�
ԭ�ӵ���������Ϊ0.977������ȷ�����⻯��ķ���ʽΪ �����⻯����ײ������ȫ�ֽ�Ϊ������������4.30 g���⻯����ײ��������������ڱ�״���µ���� L��
��������������������������ƽ�����������ȼ�ϣ�����������������������Ӧ����
�ǵ�����ˮ����������������������ɵĻ���ƽ���ǡ����ȫ��Ӧ����72.0 kgˮ���ƽ���������������Ϊ kg��
����ˮ��Һ����������NO��NO2������壬����������������Ի�������Ⱦ�����
��д���йصķ�Ӧ����ʽΪ�� �� ��
����������м��㣺
�������������������ɵ�����һ���⻯����⻯�����Է�������Ϊ43.0�����е�
ԭ�ӵ���������Ϊ0.977������ȷ�����⻯��ķ���ʽΪ �����⻯����ײ������ȫ�ֽ�Ϊ������������4.30 g���⻯����ײ��������������ڱ�״���µ���� L��
��������������������������ƽ�����������ȼ�ϣ�����������������������Ӧ����
�ǵ�����ˮ����������������������ɵĻ���ƽ���ǡ����ȫ��Ӧ����72.0 kgˮ���ƽ���������������Ϊ kg��
����ˮ��Һ����������NO��NO2������壬����������������Ի�������Ⱦ�����
��д���йصķ�Ӧ����ʽΪ�� �� ��
��1��HN3��N3H (1��) 4.48(1��)
��2��64 kg (2��)
��3��4NH3+6NO=5N2+6H2O (2��) 8NH3+6NO2=7N2+12H2O (2��)��д��NH3�� H2OҲ���֣�
�����������1��N(N)=43.0��0.977��14=3��N(H)=(43.0-14��3)��1=1������ʽΪ HN3��
n(HN3)=4.30��43=0.1mol������ԭ���غ�õ�n(H2)=0.05mol��n(N2)=0.15mol
����������0.05+0.15����22.4=4.48L
��2���÷�Ӧ����ʽΪ2N2H4+N2O4=3N2+4H2O�����ݷ���ʽ����ɵ�m(N2H4)=64kg
��3��4NH3+6NO=5N2+6H2O 8NH3+6NO2=7N2+12H2O

��ϰ��ϵ�д�
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
�����Ŀ
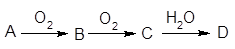
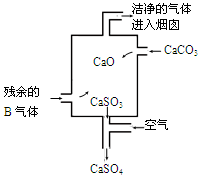
 ��4��������b�Ma��ȡֵ��Χ����֮��Ӧ����Һ�����ʼ������ʵ���������������±��У�
��4��������b�Ma��ȡֵ��Χ����֮��Ӧ����Һ�����ʼ������ʵ���������������±��У�