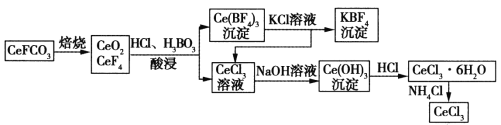
��Ŀ����
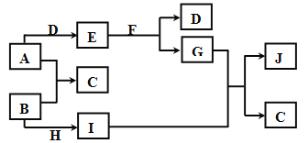
����Ŀ�����г�����A��J���ʴ�������ת����ϵʾ��ͼ����Ӧ��������ȥ������֪ͨ�������AΪ��̬�������ʣ�B��DΪ��̬�ǽ������ʣ�EΪ����ɫ���壬FΪ��ɫҺ�壬JΪ���ɫ��������ش��������⣺
��1��д���������ʵĻ�ѧʽ��H________��D__________��
��2��д������ת���Ļ�ѧ����ʽ�����ӷ���ʽ��
��E+F��D+G�Ļ�ѧ����ʽ��__________________________________��
��G+I��C+J�����ӷ���ʽ��_________________________________��
��3����Ҫ����I���ʵ���������������Լ���________________________��ʵ������Ϊ:_______________________________________
���𰸡�FeCl2 O2 2Na2O2+2 H2O=4NaOH+O2�� Fe3++3OH-=Fe��OH��3�� KSCN��NaOH ��Һ����������ɫ����
��������
��֪ͨ�������AΪ��̬�������ʣ�B��DΪ��̬�ǽ������ʣ�EΪ����ɫ���壬FΪ��ɫҺ�壬JΪ���ɫ�������ƶ�JΪFe(OH)3��FΪH2O��EΪNa2O2��GΪNaOH��DΪO2���ж�AΪNa��BΪCl2��CΪNaCl��IΪFeCl3��HΪFeCl2���ݴ˷����ش����⡣
������ת����ϵ������֪����֪ͨ�������AΪ��̬�������ʣ�B��DΪ��̬�ǽ������ʣ�EΪ����ɫ���壬FΪ��ɫҺ�壬JΪ���ɫ�������ƶ�JΪFe(OH)3��FΪH2O��EΪNa2O2��GΪNaOH��DΪO2���ж�AΪNa��BΪCl2��CΪNaCl��IΪFeCl3��HΪFeCl2��
��1��H�Ļ�ѧʽΪFeCl2�� D�Ļ�ѧʽΪO2��
��2����E+F��D+G�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����
��G+I��C+J�����ӷ���ʽΪ��Fe3++3OH��=Fe��OH��3����
��3��IΪFeCl3����Ҫ����I���ʵ���������������Լ���KSCN��NaOH��ʵ������Ϊ: ��Һ����������ɫ������