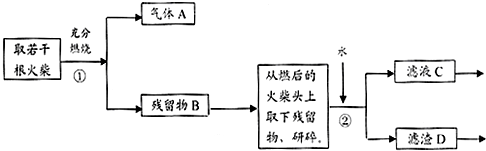
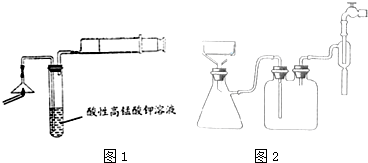
��Ŀ����
20����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�������DC�ľ���Ϊ���Ӿ��壬D��+2����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磮���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ����1��D��B�γɵĻ�����Ļ�ѧʽΪMg3N2���û�����ľ�������Ϊ���Ӿ��壮
��2��������AC2���м��Լ�������ԡ��Ǽ��ԡ�����һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��3��B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߵ�ԭ���ǰ�������֮�䡢ˮ����֮�䶼���������
��4��д������D��AC2��ȼ�յĻ�ѧ����ʽ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
��5��ECl3�γɵ������Ļ�ѧʽΪ[Cr��NH3��4��H2O��2]Cl3��
���� A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��������DC�ľ���Ϊ���Ӿ��壬D��+2����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ������д��������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��� �⣺A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��������DC�ľ���Ϊ���Ӿ��壬D��+2����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ������д��������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��1��D��B�γɵĻ�����Ļ�ѧʽΪMg3N2���û������������Ӿ��壬
�ʴ�Ϊ��Mg3N2�����Ӿ��壻
��2��������AC2��CO2��̼ԭ������ԭ��֮���γɼ��Լ���һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
�ʴ�Ϊ�����ԣ�N2O��
��3����������֮�䡢ˮ����֮�䶼����������е������ͬ����������Ԫ���⻯��ĸߣ�
�ʴ�Ϊ����������֮�䡢ˮ����֮�䶼���������
��4��Mg�ڶ�����̼��ȼ������MgO��̼����Ӧ����ʽΪ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
�ʴ�Ϊ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
��4��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��[Cr��NH3��4��H2O��2]Cl3��
���� ���⿼��ṹ����λ�ù�ϵ�ۺ�Ӧ�ã��漰�������͡���ѧ�����ȵ����塢������������ȣ��Ѷ��еȣ��Ƕ�ѧ���ۺ������Ŀ��飬�⻯��ķе������ͬ����������Ԫ���⻯��ķе�����ƶϵ�ͻ�ƿڣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 1 mol �κ����������ķ�������ΪNA�� | |
| B�� | 14 g����������Nԭ����ΪNA�� | |
| C�� | ��״���£�22.4 Lˮ������H2O������ΪNA�� | |
| D�� | �����������ķ�Ӧ�У�1 mol��ʧȥ���ӵ���ĿΪ2 NA�� |
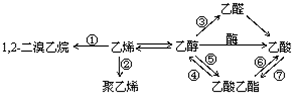
| A�� | ��Ӧ���Ǽӳɷ�Ӧ | B�� | ֻ�з�Ӧ���ǼӾ۷�Ӧ | ||
| C�� | ֻ�з�Ӧ����ȡ����Ӧ | D�� | ��Ӧ�ܢݢޢ���ȡ����Ӧ |
| A�� | BaSO4��BaCO3�ܽ��С�����ԣ�BaCO3����ת��ΪBaSO4 | |
| B�� | BaCO3��BaSO4��������ˮ�����Զ������������Լ� | |
| C�� | ��Na2CO3��Һ�м���BaCl2��Na2SO4�������ֳ�������ʱ��$\frac{c��S{O}_{4}^{2-}��}{c��C{O}_{3}^{2-}��}$=4.4��10-2 | |
| D�� | �����£�BaCO3��Ҫ��Na2SO4��Һ�п�ʼת��ΪBaSO4����Na2SO4��Ũ�ȱ��벻���� 2.2��10-6 mol•L-1 |
| A�� | ����Ͳ��ȡһ�����Һ��ʱ�����Ӷ����Ķ��� | |
| B�� | �ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ����ʽ�ζ���δ�ñ�������ϴ��������ļ�Һ��Ũ��ֵ | |
| C�� | ͬһ�ζ�ʵ�飬һ��Ҫ�����Σ�ȡ����ʵ���ƽ��ֵ���м��㣬��ijͬѧֻ����һ�� | |
| D�� | �ﵽ�ζ��յ�ʱ�����Ӷ��� |
| A�� | C��Ba2+��•C��SO42-����Ksp C��Ba2+����C��SO42-�� | |
| B�� | C��Ba2+��•C��SO42-����Ksp C��Ba2+��=C��SO42-�� | |
| C�� | C��Ba2+��=C��SO42-��=Ksp½ | |
| D�� | C��Ba2+��•C��SO42-��=Ksp C��Ba2+����C��SO42-�� |
| A�� | ���ӻ������п��ܺ����ۼ������ۻ������в������Ӽ� | |
| B�� | ���ۻ������п��ܺ����Ӽ������ӻ�������ֻ�����Ӽ� | |
| C�� | ���ɵ��ʷ��ӵ���һ�����й��ۼ� | |
| D�� | ˫ԭ�ӷ����еĹ��ۼ���һ���ǷǼ��Լ� |