��Ŀ����
15��ij��ȤС��Ϊ��֤�ճ������õĻ��ͷ�Ϻ���KClO3��MnO2��S�����ʣ����������ʵ������ͼ��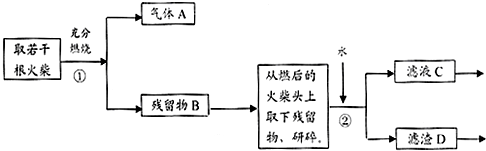
��ش��������⣺
��1��Ϊ��֤����A������ͼ1��ʾװ�ý���ʵ�飺���ܹ۲쵽���Ը��������Һ��ɫ������֤�����ͷ�Ϻ���SԪ�أ���д������Aʹ���Ը��������Һ��ɫ�����ӷ���ʽ5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+��
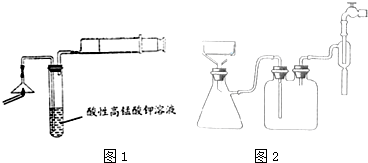
��2������ڵ�ʵ�����װ����ͼ2��ʾ���ò����������Ǽ�ѹ�������ŵ��ǹ����ٶȼӿ죬�õ��ϸ���Ĺ������ʣ�
��3��ָ��ͼ�еĴ���
����ٰ�ȫƿ�г��ܺͶ̹ܵ�����˳��Ū����
����ڲ���©���ľ���б��û�к�����ƿ֧�ܿ���ԣ�
�����-�����м�������д��������һ��Ҫ������
���� ��1������AΪ����������������л�ԭ�ԣ��ܹ������Ը��������Һ���������ᣬ�ݴ�д����Ӧ�����ӷ���ʽ��
��2����װ�������Ǽ�ѹ���ˣ������ԭ��������ѹǿ����й��ˣ��ݴ��ж����ŵ㣻
��3������ͼ1��ͼ2װ�ü���ȷ�IJ��������жϣ�
��� �⣺��1������к��е�Sȼ�պ����ɶ����������壬���������ܹ������Ը�������������Ӷ��������Ը��������Һ��ɫ����Ӧ�����ӷ���ʽΪ��5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+��
�ʴ�Ϊ��5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+��
��2����װ�������Ǽ�ѹ���ˣ��乤��ԭ���ǵ�������ˮ��ͷʱ��װ���ڲ��Ŀ���������ˮ�����ߣ�����װ���ڲ�������ѹ���Ӷ��ӿ�����ٶȣ��õ��ϸ���Ĺ������ʣ��������ŵ�Ϊ�������ٶȼӿ죬�õ��ϸ���Ĺ������ʣ�
�ʴ�Ϊ����ѹ���ˣ�����˻����ˣ��������ٶȼӿ죬�õ��ϸ���Ĺ������ʣ�
��3������ͼʾ��֪��ͼ1�д���Ϊ����ȫƿ�г��ܺͶ̹ܵ�����˳��Ū����ͼ2�еĴ���Ϊ������©���ľ���б��û�к�����ƿ֧�ܿ���ԣ�
�ʴ�Ϊ����ȫƿ�г��ܺͶ̹ܵ�����˳��Ū��������©���ľ���б��û�к�����ƿ֧�ܿ���ԣ�
���� ���⿼����̽��������ɵ�ʵ�飬��Ŀ�Ѷ��еȣ���ȷʵ��ԭ��Ϊ���ؼ���ע�����ջ�ѧʵ�����������������ȷ������������ȷʹ�÷���������������ѧ���ķ�����������ѧʵ��������

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�| A�� | ����������Һ | B�� | ϡ���� | C�� | Ũ���� | D�� | ϡ���� |
| A�� | 2Al��s��+2NaOH��aq��+2H2O��1���T2NaAlO2��aq��+3H2��g������H��O | |
| B�� | Ba��OH��2•8H2O��s��+2NH4Cl��s���TBaCl2��aq��+2NH3•H2O��aq��+8H2O��1������H��0 | |
| C�� | C3H8��g��+5O2��g����4H2O ��l��+3CO2��g������H��0 | |
| D�� | FeCl3��aq��ʮ3H2O��1��?Fe��OH��3��s��+3HCl��aq������H��0 |
| A�� | ������ʱ��ˮ���ռ���������ֵ������������ɿ��Թ��ϵ���Ƥ�� | |
| B�� | �����巢��װ����ֱ�ӵ�ȼ��������ʱ�������ȼ����������Ĵ��� | |
| C�� | ʵ��ʱ�����еķ���ͨ��ͨ����ų�ʵ���ң�������Ⱦʵ���� | |
| D�� | ���Թ��е�Һ�����ʱ��ʱ�ƶ��Թܻ�������Ƭ�����Ⱪ������ |
| A�� | Ũ��������ܷų����� | |
| B�� | ŨHNO3��Ũ��������Ƴ���ˮ | |
| C�� | HNO3��������Ӧ�����ɼ�̬��ͬ�������� | |
| D�� | ŨHNO3��ͭ���ҷ�Ӧ�Ҳ������ |
| A�� |  �ⶨ��Ӧ���� | B�� |  �ⶨ�к��� | ||
| C�� | 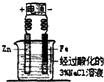 �������������������� | D�� |  ����������ʴʵ�� |
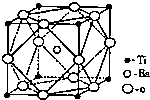 �Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺
�Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺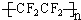 ���ͼӾ۷�Ӧ
���ͼӾ۷�Ӧ +3NaOH$\stackrel{��}{��}$3C17H35COONa+
+3NaOH$\stackrel{��}{��}$3C17H35COONa+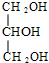 ����ˮ�ⷴӦ
����ˮ�ⷴӦ ����ȡ����Ӧ��
����ȡ����Ӧ��