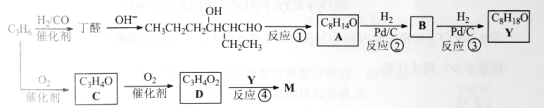
��Ŀ����
����Ŀ��ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ����м��ϡ���ᡢ����������Һ��
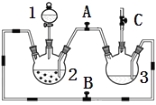
��1��ϡ����Ӧ���� ������д������������
��2����ʵ��ͨ������A��B��C�������أ��������еĿ����ž����ٹرտ��� ������ �Ϳɹ۲쵽��������������ɫ���Է���ʵ�鿪ʼʱ�ž�װ���п���������
��3��ʵ��ʱΪ��ֹ����2������ͨ�����ܽ�������3�У��ɲ�ȡ�Ĵ�ʩ�� ��
��4����FeSO4��Һ�м�����NH4��2SO4������Ʊ�Ħ���ξ���[��NH4��2SO4��FeSO4��6H2O] ����Է�������392�����þ����һ���������ȶ������ױ�������������ˮ���������Ҵ���
��Ϊϴ����NH4��2SO4��FeSO4��6H2O�ֲ�Ʒ�����з���������ʵ���
A������ˮϴ B��������ˮϴ��������ˮ�Ҵ�ϴ
C����30%���Ҵ���Һϴ D����90%���Ҵ���Һϴ
��Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ȡa g��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊc mol��L-1������KMnO4��Һ�ζ���ÿ����ȡ����Һ�����Ϊ25��00mL��ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ����� | ������ |
���ĸ��������Һ���/mL | 25��52[ | 25��02 | 24��98 |
�ζ������з�����Ӧ�����ӷ���ʽΪ
�ζ��յ��������
ͨ��ʵ�����ݼ���ĸò�Ʒ����Ϊ ������ĸac��ʾ�����ϱ��е�һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
D�������Ը�����ر�Һ����ʱ��������в��ֱ��ʣ�Ũ�Ƚ���
���𰸡���1����Һ©��
��2��B A����AC������ ��ֹ���ɵ�����������������
��3�������ۻ�������������
��4����D ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
���һ�ε��룬��Һ����ɫ��Ϊdz��ɫ����30s����ɫ ![]() ��100% BC
��100% BC
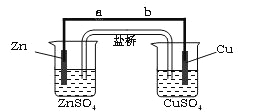
��������
�����������1����Һ©������������ϡ��������٣�������ƿ�Ƿ�Ӧװ�ã�����ϡ����Ӧ��������1������Һ©���С�
��2������Fe��ϡ���ᷴӦ���ɵ�H2�ų�װ���ڵĿ����رտ���B������������ƿ�����岻����ͨ��������ƿ2������������ѹǿ������A��AC����Ϊѹǿ�������ƿ2�ڵ�FeSO4��Һ����������ƿ3�У�FeSO4��NaOH��Ӧ����Fe��OH��2����; Fe��OH��2���ױ�����������ʵ�鿪ʼʱ�ž�װ���п����ɷ�ֹ���ɵ�������������������
��3��ʵ��ʱΪ��ֹ����2������ͨ�����ܽ�������3�У��ɲ�ȡ�Ĵ�ʩ�������ۻ�������������
��4������Ϊ��NH4��2SO4��FeSO4��6H2O����������ˮ���������Ҵ���Ϊ�˷�ֹ�������ʧ��������90%���Ҵ���Һϴ����D����ȷ�� ��MnO4_�����������°�Fe2+����ΪFe3+��MnԪ��ת��ΪMn2+�����ݻ��ϼ۵ı仯��ƽ�ɵ����ӷ���ʽ:MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O;�ζ��յ�ʱǡ����ȫ��Ӧ�������ٵ���KMnO4��Һ����Һ������ɫ�����Եζ��յ��������:���һ�ε��룬��Һ����ɫ��Ϊdz��ɫ����30s����ɫ;��һ�εζ��Ľ���������������ϴ���ȥ���ڶ��κ͵��������ĸ��������Һ���ƽ��ֵΪ25mL��n[��NH4��2SO4��FeSO4��6H2O]= n��Fe2+��= 5n��MnO4���� =5��0��025c= 0��125c mol����Ʒ����NH4��2SO4��FeSO4��6H2O���ʵ���Ϊ0��125c mol��500mL/25mL=2��5cmol�����Բ�Ʒ���� 2��5cmol��392g/mol��a g��100% =![]() ��100%��A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ���������ɸ��������Һ������������ƫС���ᵼ����Ʒ����ƫС������;B���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ���ɸ��������Һ������������ƫ�ᵼ����Ʒ����ƫ����ȷ;C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����Ʒ������ƿ���ϣ����ĸ��������Һƫ�࣬�ᵼ����Ʒ����ƫ����ȷ;D������ʹ�õĸ��������Һ��ͬ�������ܳ���һ�εĽ�����Դ����������Σ�������
��100%��A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ���������ɸ��������Һ������������ƫС���ᵼ����Ʒ����ƫС������;B���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ���ɸ��������Һ������������ƫ�ᵼ����Ʒ����ƫ����ȷ;C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����Ʒ������ƿ���ϣ����ĸ��������Һƫ�࣬�ᵼ����Ʒ����ƫ����ȷ;D������ʹ�õĸ��������Һ��ͬ�������ܳ���һ�εĽ�����Դ����������Σ�������

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�