��Ŀ����
14�����и��������ڸ���������һ���ܴ���������ǣ�������| A�� | ��c��HCO${\;}_{3}^{-}$��=0.1 mol•L-1����Һ�У�NH${\;}_{4}^{+}$��Al3+��Cl-��NO${\;}_{3}^{-}$ | |
| B�� | �д���NO${\;}_{3}^{-}$���ڵ�ǿ������Һ�У�NH${\;}_{4}^{+}$��Ba2+��Fe3+��Cl- | |
| C�� | ��ˮ�������c��H+��=1��10-12mol•L-1����Һ�У�Na+��Al3+��CH3COO-��I- | |
| D�� | ��ʹpH��ֽ������Һ�У�ClO-��S2-��Na+��K+ |
���� A������֮����ٽ�ˮ�⣻
B����������֮�䲻��Ӧ��
C����ˮ�������c��H+��=1��10-12mol•L-1����Һ��Ϊ������Һ��
D����ʹpH��ֽ������Һ�������ԣ�����֮�䷢��������ԭ��Ӧ��
��� �⣺A��HCO3-��Al3+����˫ˮ�ⷴӦ�����ܴ������棬��A����
B����������֮�䲻��Ӧ���ɴ������棬��B��ȷ��
C����Һ�����Ի���ԣ�Al3+��OH-���ܴ������棬CH3COO-��H+���ܴ������棬��C����
D��������Һ��S2-��ClO-����������ԭ��Ӧ�����ܴ������棬��D����
��ѡB��
���� ���⿼�����ӵĹ��棬Ϊ��Ƶ���㣬����ϰ���е���Ϣ����������֮��ķ�ӦΪ���Ĺؼ�������������ԭ��Ӧ��ˮ�ⷴӦ�����ֽⷴӦ�����ӹ��濼�飬�ۺ��Խ�ǿ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ
4������˵����ȷ���ǣ�������
| A�� | ��������ԭ��Ӧ�У��������뻹ԭ����������ͬһ������ | |
| B�� | �������ڷ�Ӧ�б���������ԭ���ڷ�Ӧ�б���ԭ | |
| C�� | ������ֻ�������ԣ�������ֻ�л�ԭ�� | |
| D�� | �����������д����м��̬��Ԫ�أ�������ʿ��ܼȾ����������־��л�ԭ�� |
5�������л���Ӧ�У���һ�ַ�Ӧ�������������ַ�Ӧ���Ͳ�ͬ���ǣ�������
| A�� | CH3COOH+CH3CH2OH$��_{����}^{Ũ����}$CH3COOCH2CH33+H2O | |
| B�� | 2CH3CH2OH+O2 $��_{��}^{����}$ 2CH3CHO+2H2O | |
| C�� | CH4+Cl2$\stackrel{��}{��}$CH3Cl+HCl | |
| D�� | 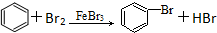 |
2������ʵ���������ȷ���ǣ�������
| A�� | �����Ȼ�����Һʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ���� | |
| B�� | ����ʯ��ʱ��Ӧʹ�¶ȼ�ˮ�������Һ������ | |
| C�� | ��Һ����ʱ���²�Һ��ӷ�Һ©���¿ڷų����ϲ�Һ����Ͽڷŵ���һ���ձ� | |
| D�� | ������������ӵIJ������ȼ������ữ�ټ����Ȼ�����Һ |
9������������ʾ���淴ӦN2+3H2?2NH3һ������ƽ��״̬���ǣ�������
| A�� | N2��H2��NH3�İٷֺ������ | |
| B�� | ��λʱ�䣬����a mol N2��ͬʱ����3mol H2 | |
| C�� | ��λʱ�䣬����a molN2��ͬʱ����3a mol H2 | |
| D�� | ��Ӧ���ڶ��ݵ������н��У��¶�һ��ʱ��ѹǿ����ʱ��ı� |
19��2molNaOH���200mL��Һ�������ʵ���Ũ��Ϊ��������
| A�� | 2mol/L | B�� | 10 mol/L | C�� | 0.2 mol/L | D�� | 0.1 mol/L |
11��CuCl2���������ϡ�ľ�ķ����ȹ�ҵ����������������ýȾ������������ҵ���ô��Ƶ�����ͭ��ĩ��������FeO��SiO2������ȡ��ˮCuCl2���������£�

��֪��Fe3+��Fe2+��Cu2+ת��Ϊ��Ӧ�����������ʼ�����ͳ�����ȫʱ��pH���±���
��1���ڢڲ���Ӧ�����ӷ���ʽΪ2Fe2++Cl2�T2Fe3++2Cl-��
��2������A�ijɷ���SiO2���ѧʽ����������ҺC�е�Fe3+�����Ƿ�����ķ�����ȡ��ҺC�������Թ��У��μӼ���KSCN��Һ������Һ��ΪѪ��ɫ��֤����ҺC�е�Fe3+����δ��������֮�ѳ�����
��3���ڢܲ�����������Ҫ������������̨������Ȧ��������ǯ��ʯ�������ƾ��ơ���������������Ҫ���Ȼ���������������ȡ��ˮ�Ȼ�ͭ��ԭ����HCl����CuCl2ˮ�⣮
��4����ȡ30.250g���Ƶõ���ˮCuCl2��Ʒ��������FeCl3���ʣ�������ˮ�У�������������۳�ַ�Ӧ����ˣ���250mL��Һ����ȡ25.00mL��Һ����ƿ�У���0.100mol?L-1����KMnO4��Һ�ζ����յ㣬����KMnO4��Һ���ƽ��Ϊ46.00mL�������ˮCuCl2��Ʒ��CuCl2����������Ϊ0.89����С����ʾ������������2λС������

��֪��Fe3+��Fe2+��Cu2+ת��Ϊ��Ӧ�����������ʼ�����ͳ�����ȫʱ��pH���±���
| ���ӷ��� | Fe3+ | Fe2+ | Cu2+ |
| �������↑ʼ����ʱ��pH | 2.5 | 7.0 | 4.7 |
| ����������ȫ����ʱ��pH | 3.2 | 9.7 | 6.7 |
��2������A�ijɷ���SiO2���ѧʽ����������ҺC�е�Fe3+�����Ƿ�����ķ�����ȡ��ҺC�������Թ��У��μӼ���KSCN��Һ������Һ��ΪѪ��ɫ��֤����ҺC�е�Fe3+����δ��������֮�ѳ�����
��3���ڢܲ�����������Ҫ������������̨������Ȧ��������ǯ��ʯ�������ƾ��ơ���������������Ҫ���Ȼ���������������ȡ��ˮ�Ȼ�ͭ��ԭ����HCl����CuCl2ˮ�⣮
��4����ȡ30.250g���Ƶõ���ˮCuCl2��Ʒ��������FeCl3���ʣ�������ˮ�У�������������۳�ַ�Ӧ����ˣ���250mL��Һ����ȡ25.00mL��Һ����ƿ�У���0.100mol?L-1����KMnO4��Һ�ζ����յ㣬����KMnO4��Һ���ƽ��Ϊ46.00mL�������ˮCuCl2��Ʒ��CuCl2����������Ϊ0.89����С����ʾ������������2λС������
