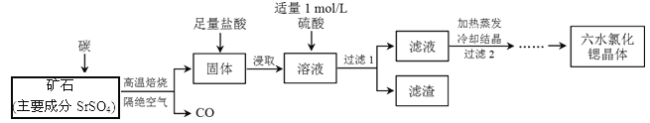
��Ŀ����
����Ŀ��X��Q��R��Z��T��U�ֱ����ԭ���������������Ԫ�ء�X��ԭ�Ӱ뾶��С��Ԫ�أ���������Qԭ�Ӻ��е�δ�ɶԵ�������࣬R��T��ͬ�壬T��ԭ��������R��������Z�ĵ��ʵ�ͬ�������۵���ߣ�U5+�ĺ�������Ų����Ԫ����ͬ��
��1��U��̬ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ ��
��2����(QX4)2TR4�ľ����д��ڵĻ�ѧ�������� ��
a�����Ӽ� b�����ۼ� c����λ�� d��������
��3��Q��R��T����ӦԪ�صĵ�һ����������С��˳���� (��Ԫ�ط���)��
��4��TR2�� ���ӣ�����ԡ��Ǽ��ԡ�����ZR2����ṹ��ͼI��6g ZR2������Z��R������ĿΪ ��
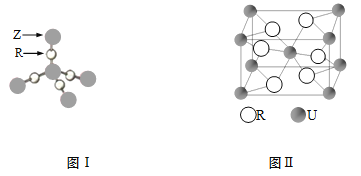
��5��U��R�γɵĻ�����ľ����ṹ��ͼII��ʾ���û�����Ļ�ѧʽΪ ��
���𰸡���1��
��2��abc
��3��N��O��S
��4��������0.4mol
��5��VO2
��������
���������X��Q��R��Z��T��U�ֱ����ԭ���������������Ԫ�أ�X��ԭ�Ӱ뾶��С��Ԫ�أ���XΪHԪ�أ�R��T��ͬ�壬T��ԭ��������R����������RΪOԪ�ء�TΪSԪ�أ���������Qԭ�Ӻ��е�δ�ɶԵ�������࣬����VA�壬ԭ������С��������QΪNԪ�أ�Z�ĵ��ʵ�ͬ�������۵���ߣ�ԭ��������������С����ZΪSi��U5+�ĺ�������Ų����Ԫ����ͬ��ԭ�Ӻ��������Ϊ18+5=23����UΪVԪ����
��1��U��̬ԭ�Ӻ��������Ϊ23��ԭ�ӽṹʾ��ͼΪ��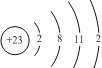 ��
��
�ʴ�Ϊ�� ��
��
��2���ڣ�NH4��2SO4��笠����������������֮���γ����Ӽ���笠������к��й��ۼ�����λ���������������Ҳ���й��ۼ���û�н�������
��ѡ��abc��
��3��ͬ�������϶��µ�һ�����ܼ�С����Ԫ��2p���Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ��ʵ�һ�������ɴ�С��˳���ǣ�N��O��S��
�ʴ�Ϊ��N��O��S��
��4��SO2������Sԭ��û��ȫ���ɼ������ڼ��Է�����SiO2������Siԭ�������յ�4��Oԭ���γ�4��Si-O����6g SiO2�����ʵ���Ϊ![]() =0.1mol������Si-O������ĿΪ0.4mol��
=0.1mol������Si-O������ĿΪ0.4mol��
�ʴ�Ϊ�����ԣ�0.4mol��
��5��������Vԭ����ĿΪ1+8��![]() =2��Oԭ����ĿΪ2+4��
=2��Oԭ����ĿΪ2+4��![]() =4���û�����Ļ�ѧʽΪVO2��
=4���û�����Ļ�ѧʽΪVO2��
�ʴ�Ϊ��VO2��