��Ŀ����
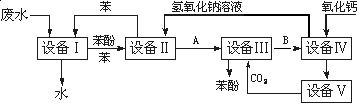
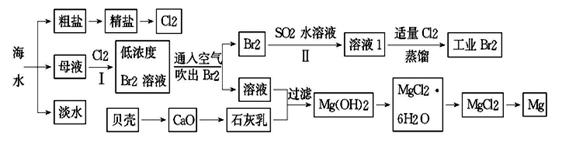
��֪��BaSO4(s) + 4C(s) 4CO(g) + BaS(s)��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��Ϊԭ�ϣ�ͨ���������������Ȼ������壨BaCl2��nH2O����
4CO(g) + BaS(s)��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��Ϊԭ�ϣ�ͨ���������������Ȼ������壨BaCl2��nH2O����

�ش��������⣺
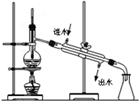
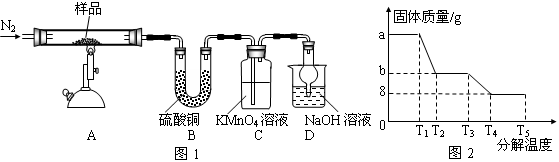
��1��������A�Ļ�ѧʽ��_________������ʵ���ҽ��б���ʱ��������������Ĵ���������
a.��NaOH��Һ���� b.��Ũ�������� c.��ȼ
��2���õ�λ�����Һ����������������������ʾ��Ũ�Ƚ�����-���Ũ�ȣ�������g/L��ʾ,����38%��Ũ�������ƺ�����109.5g/L��ϡ����500mL,����Ҫ�IJ����������˲��������� ��
��3��������Ӧ�����ӵ��Լ�R�����������Լ��е�
a.NaOH��Һ b.BaO���� c.��ˮ d.��ʯ��
֤�������Ѿ���ȫ�ķ�����________________________________________________________��
��4�����һ��ʵ��ȷ����Ʒ�Ȼ������壨BaCl2��nH2O���е�nֵ����������ʵ�鲽�裺
�ٳ�����Ʒ��_______ ������_________�����������ƣ�����ȴ �ܳ��� �ݺ��ز�����
���ز�����ָ____________________________________________ _��
�ڢ۲���Ʒ֮���Է��ڸ������н���ʵ���ԭ���� ��
��5�����ؾ�ʯ����̼�Լ��Ȼ��ƹ�ͬ���գ�����ֱ�ӵõ��Ȼ������÷�Ӧ�Ļ�ѧ����Ϊ
BaSO4+ 4C+CaCl2 4CO + CaS+ BaCl2�������������дӱ��պ�Ĺ����з���õ��Ȼ��������ʵ�����̵���ƣ���֪�Ʋ�����ˮ�����������ᣩ��
4CO + CaS+ BaCl2�������������дӱ��պ�Ĺ����з���õ��Ȼ��������ʵ�����̵���ƣ���֪�Ʋ�����ˮ�����������ᣩ��
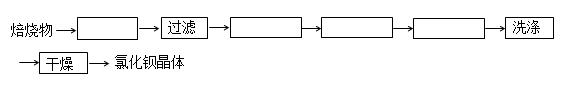
����������д�������ƣ�
 4CO(g) + BaS(s)��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��Ϊԭ�ϣ�ͨ���������������Ȼ������壨BaCl2��nH2O����
4CO(g) + BaS(s)��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��Ϊԭ�ϣ�ͨ���������������Ȼ������壨BaCl2��nH2O����
�ش��������⣺
��1��������A�Ļ�ѧʽ��_________������ʵ���ҽ��б���ʱ��������������Ĵ���������
a.��NaOH��Һ���� b.��Ũ�������� c.��ȼ
��2���õ�λ�����Һ����������������������ʾ��Ũ�Ƚ�����-���Ũ�ȣ�������g/L��ʾ,����38%��Ũ�������ƺ�����109.5g/L��ϡ����500mL,����Ҫ�IJ����������˲��������� ��
��3��������Ӧ�����ӵ��Լ�R�����������Լ��е�
a.NaOH��Һ b.BaO���� c.��ˮ d.��ʯ��
֤�������Ѿ���ȫ�ķ�����________________________________________________________��
��4�����һ��ʵ��ȷ����Ʒ�Ȼ������壨BaCl2��nH2O���е�nֵ����������ʵ�鲽�裺
�ٳ�����Ʒ��_______ ������_________�����������ƣ�����ȴ �ܳ��� �ݺ��ز�����
���ز�����ָ____________________________________________ _��
�ڢ۲���Ʒ֮���Է��ڸ������н���ʵ���ԭ���� ��
��5�����ؾ�ʯ����̼�Լ��Ȼ��ƹ�ͬ���գ�����ֱ�ӵõ��Ȼ������÷�Ӧ�Ļ�ѧ����Ϊ
BaSO4+ 4C+CaCl2
 4CO + CaS+ BaCl2�������������дӱ��պ�Ĺ����з���õ��Ȼ��������ʵ�����̵���ƣ���֪�Ʋ�����ˮ�����������ᣩ��
4CO + CaS+ BaCl2�������������дӱ��պ�Ĺ����з���õ��Ȼ��������ʵ�����̵���ƣ���֪�Ʋ�����ˮ�����������ᣩ��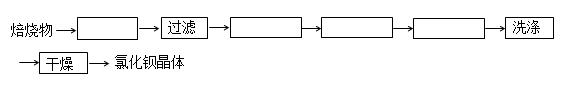
����������д�������ƣ�
��1��SiO2 c
��2���ձ� 500mL����ƿ ��ͷ�ι�
��3��b��ȡ�ϲ���Һ��С�Թ��У���������������Һ��������������˵��������ȫ��
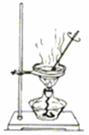
��4�����ȡ����������ٽ��м��ȡ���ȴ��������ֱ���������γ����Ľ��������0.001gΪֹ����ֹ��ȴ���������տ����е�ˮ���������ʵ����
��5��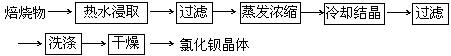
��2���ձ� 500mL����ƿ ��ͷ�ι�
��3��b��ȡ�ϲ���Һ��С�Թ��У���������������Һ��������������˵��������ȫ��
��4�����ȡ����������ٽ��м��ȡ���ȴ��������ֱ���������γ����Ľ��������0.001gΪֹ����ֹ��ȴ���������տ����е�ˮ���������ʵ����
��5��

�����������1����ҵ�����ؾ�ʯ�����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��������ݵ���SiO2��������֪�������Ļ�ѧ����ʽ��֪������������ΪCO������CO�ķ����ǽ�CO��ȼ��
��2��������Һ���貣�������У��ձ���500mL����ƿ����ͷ�ιܡ���������
��3��BaO��ˮ��Ӧ���������������ܹ��������ӳ������Ҳ������µ����ʣ���ѡ�õ��Լ�ΪBaO���壻֤�������Ѿ���ȫ�ķ�����ȡ�ϲ���Һ��С�Թ��У���������������Һ��������������˵��������ȫ��
��4����ʵ���ԭ�������ü��Ⱥ������ǰ�����������Ȼ��������е�ˮ�����ʵ�����ʵ��������¢ٳ�����Ʒ�ڼ��Ȣ۸���������ȴ �ܳ��� �ݺ��ز��������ز�����ֱָ���������γ����Ľ��������0.001gΪֹ���ڢ۲���Ʒ֮���Է��ڸ������н���ʵ���ԭ����Ϊ�˷�ֹ��ȴ���������տ����е�ˮ���������ʵ����
��5������BaSO4+ 4C+CaCl2
 4CO + CaS+ BaCl2��֪��Ӧ��һ����̼�ݳ���Ҫ��ӱ��պ�Ĺ����з���õ��Ȼ���������Ҫ�ӻ����Һ�з���CaS����CaS����ˮ����ֱ�ӹ��˼��ɵõ�BaCl2��Һ��ʵ��������£�
4CO + CaS+ BaCl2��֪��Ӧ��һ����̼�ݳ���Ҫ��ӱ��պ�Ĺ����з���õ��Ȼ���������Ҫ�ӻ����Һ�з���CaS����CaS����ˮ����ֱ�ӹ��˼��ɵõ�BaCl2��Һ��ʵ��������£�

��ϰ��ϵ�д�
 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
�����Ŀ