��Ŀ����
����Ŀ��AA705�Ͻ�(��Al��Zn��Mg��Cu)�������һ����̣���������Ϊ�ֵ�����֮һ���ѱ����ڷɻ������ͻ����������ֻ�����ϵȡ������ֺϽ���ѱ����ӡ������ѧ�ҽ�̼����������(��С��Ϊʮ�ڷ�֮һ��)ע��AA7075�ĺ�˿�ڣ�����Щ�������䵱���Ӽ�֮��������ϡ�ע�����������ӵ���亸˿Ҳ���Ը����������������Ժ��ӵĽ����ͽ����Ͻ𡣻ش��������⣺
(1)��̬ͭԭ�ӵļ۲�����Ų�ʽΪ__________��
(2)��������ijԪ�ص�ǰ5�����ӵĵ�������ͼ1��ʾ����Ԫ����_____(��Ԫ�ط���)���ж�������_______��

(3)CN����NH3��H2O��OH�������嶼����Zn2+�γ������ӡ�1mol [Zn(NH3)4]2+��___ mol�������������ӵ���λ��Ϊ_____��
(4)��þ�Ͻ������ʴ��Ʋ���,ԭ��λ�����ĺͶ��㣬�侧����ͼ2��ʾ��1����ԭ����Χ��_____��þԭ������ҵȾ��롣
(5)�ڶ������Ѻ��������£����״��ɱ������ɱ���ȩ��

�ٱ��״���Cԭ���ӻ�������__________��
�ڱ��״��ķе���ڱ���ȩ����ԭ����__________��
(6)�Ѿ���������Ʒ������ͼ��ʾ��

����ͼ3��ʾ�������Ŀռ�������Ϊ______(�ú�����ʽ�ӱ�ʾ)��
����֪ͼ4���������߳�Ϊx cm����Ϊy cm�����Ѿ����ܶ�ΪD g��cm-3��NAΪ______mol��1(�ú�x y��D��ʽ�ӱ�ʾ)��
���𰸡�3d104s1 Mg I3��I2��5���࣬˵���������2�����ӣ� 16 4 8 sp2��sp3 ���״����Ӽ�������������ȩ���Ӽ䲻������� ![]()
![]()
��������
��1��ͭԭ����29�����ӣ���3d��4s�ܼ��ϵĵ���Ϊ��۵��ӣ�
(2)���ݵ����ܵ�ͻ���ж��������ӣ���ϸ�Ԫ��Ϊ��������Ԫ�ط�����
(3)���е���������λ����Ϊ������˫������һ��Ϊ������ÿ��NH3�����к���3��N-H��������ԭ��Zn���ĸ�Nԭ��֮�������λ����
(4)������ÿ�������4�������ϵ�þԭ�Ӻ������ϵ�þԭ�ӵ�Alԭ�ӵľ�������������
(5)�ٱ�����̼ԭ���γ�3�����õ��Ӷԣ���-CH2OH��Cԭ���γ�4�����õ��Ӷԣ�
�ڷ��Ӽ����������ʵķе�Ӱ��ϴ��״����Ӽ���������
(6)����ͼ3��֪����������ԭ�ӵ���ĿΪ1+8��![]() =2����ԭ�Ӱ뾶Ϊr�����ĶԽ���Ϊ4r�������ı߳�Ϊ
=2����ԭ�Ӱ뾶Ϊr�����ĶԽ���Ϊ4r�������ı߳�Ϊ![]() ���ɴ˼���ռ������ʣ�
���ɴ˼���ռ������ʣ�
��ͼ4��������ԭ�ӵ���ĿΪ3+2��![]() +12��
+12��![]() =6������������Ϊ
=6������������Ϊ![]() g���������߳�Ϊx cm����Ϊy cm���������Ϊ
g���������߳�Ϊx cm����Ϊy cm���������Ϊ![]() x2ycm3���ٽ�Ͼ������ܶȼ���NA��
x2ycm3���ٽ�Ͼ������ܶȼ���NA��
��1��ͭԭ����29�����ӣ���3d��4s�ܼ��ϵĵ���Ϊ��۵��ӣ���۵����Ų�ʽΪ3d104s1��
(2)��ͼ1��֪������I3��I2��5���࣬˵���������2�����ӣ���ϸ�Ԫ���ǵ�������Ԫ�أ����Ԫ��Ϊ�������ڵڢ�AԪ�أ���Ԫ��Ϊþ��Ԫ�ط���ΪMg��
(3)���е���������λ����Ϊ������˫������һ��Ϊ������ÿ��NH3�����к���3��N-H��������ԭ��Zn���ĸ�Nԭ��֮�������λ������1mol [Zn(NH3)4]2+����4+3��4��mol=16mol��������λ��ΪNH3����������Zn2+����λ��Ϊ4��
(4)������ÿ�������4�������ϵ�þԭ�Ӻ������ϵ�þԭ�ӵ�Alԭ�ӵľ���������������ÿ����ԭ����Χ���������þԭ����8����
(5)�ٱ�����̼ԭ���γ�3�����õ��Ӷԣ�̼ԭ�ӵ��ӻ�������sp2����-CH2OH��Cԭ���γ�4�����õ��Ӷԣ�̼ԭ�ӵ��ӻ�������sp3��
�ڱ��״����Ӽ���������������ȩ���Ӽ䲻������������±��״��ķе����Աȱ���ȩ�ߣ�
(6)����ͼ3��֪����������ԭ�ӵ���ĿΪ1+8��![]() =2����ԭ�Ӱ뾶Ϊr�����ĶԽ���Ϊ4r�������ı߳�Ϊ
=2����ԭ�Ӱ뾶Ϊr�����ĶԽ���Ϊ4r�������ı߳�Ϊ![]() ����ռ�������Ϊ
����ռ�������Ϊ =
=![]() ��
��
�ڢ�ͼ4��������ԭ�ӵ���ĿΪ3+2��![]() +12��
+12��![]() =6������������Ϊ
=6������������Ϊ![]() gspan>���������߳�Ϊx cm����Ϊy cm���������Ϊ
gspan>���������߳�Ϊx cm����Ϊy cm���������Ϊ![]() x2ycm3����D=
x2ycm3����D=![]() g��
g��![]() x2ycm3���ɴ˼����NA=
x2ycm3���ɴ˼����NA=![]() mol��1��
mol��1��

����Ŀ���Ҷ����ǹ�ҵ�������л�ԭ�ϣ���������ȡ��֯��ҵ���Ҷ�ȩ�ͻ�ױƷ��ҵ����ȩ�ᡣ
���Ҷ���(HOCH2CH2OH)�������������ȡ�Ҷ�ȩ(OHC-CHO)����Ҫ��ӦΪ��HOCH2CH2OH(g)+O2(g)![]() OHC��CHO(g)+2H2O(g) H
OHC��CHO(g)+2H2O(g) H
��ѧ�� | O��H | C��H | C��O | C=O | O=O | C��C |
���ܣ�kJ��mol-1�� | 436 | 413 | 356 | 745 | 493 | 346 |
��H=_____kJ��mol-1��
�ڵ�ԭ������������(�������Ҷ��������ʵ���֮��)һ��ʱ���Ҷ�ȩ������CO2�IJ����뷴Ӧ�¶ȵĹ�ϵ��ͼ��ʾ����Ӧ��Ӧ���Ƶ������¶���___(����ĸ)��
a.����450��
b.450��~490��
c.����495��

p��m��n������____��ƽ��㣬__���淴Ӧ�������ĵ㡣
���¶ȳ���495��ʱ���Ҷ�ȩ�IJ���ֱ���½���ԭ����___��
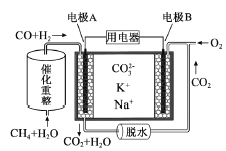
��2�����Ҷ�������Ϊ�Ҷ��ᣬ�������Ҷ���(HOOCCOOH)ͨ������Ʊ���ȩ�ᣬ�����ĵ缫��ӦʽΪ____��
��3��Һ�������Ʊ���ȩ���ǽ����о����ȵ㡣��25LijŨ�ȵ��Ҷ�ȩ��Һ�У����������Ĵ���V2O5/C����0.1mol��L-1������ͨ�������������Һ���Ҷ�ȩ��Ũ�ȡ���Һ��pH��ʱ��仯�Ĺ�ϵ��ͼ��ʾ��

��ͼ������___(����a������b��)��ʾ��Һ��pHֵ��ʱ��仯�����ߡ�
��V2O5/C��ʾ����������������̼��ά�ϣ���Ŀ����___��
��д���Ʊ���ȩ��(HOC��COOH)�Ļ�ѧ��Ӧ����ʽ��____������ͼ���������8h����ȩ���ƽ����������v(HOC��COOH)=____����ʵ����������ȩ��ĵ��볣��Ka=___��