��Ŀ����
��һƿ��ɫ��Һ�����ܺ���Na+��K+��Al3+��Mg2+��NH4+��Cl����SO42����HCO3����MnO4�������еļ��֡�Ϊȷ����ɷ֣���������ʵ�飺��ȡ������Һ�����������Na2O2���壬������ɫ��ζ������Ͱ�ɫ�����Ұ�ɫ������������ֲ����ܽ⣻����ȡ������Һ������HNO3�ữ��Ba(NO3)2��Һ���а�ɫ�������������ýྻ�IJ�˿պȡԭ��Һ�ھƾ��ƻ��������գ��۲쵽��ɫ���档�����ƶ���ȷ���� �� ��
| A���϶���Na+��Al3+��Mg2+��SO42�� | B���϶���Na+��Mg2+��Al3+��HCO3�� |
| C���϶�û��K+��HCO3����MnO4�� | D���϶�û��K+��NH4+��Cl�� |
A
���������������ɫ��Һ�϶�û��MnO4������ȡ������Һ����������Na2O2���壬�����������Ǻ�ˮ��Ӧ�����������ƺ�������������ɫ��ζ��������������һ�����ǰ�������ʱ��ɫ�������֣��ټ���������NaOH��Һ���ɫ���������ܽ⣬������ijɷ���������þ��������������֤������һ������þ���Ӻ������ӣ�һ��������笠����ӡ�̼��������ӣ��������Ӳ����棩����ȡ������Һ������HNO3�ữ��Ba(NO3)2��Һ���а�ɫ��������������������ӷ�Ӧ���ɰ�ɫ����������İ�ɫ���������ᱵ������֤��һ��������������ӡ���ѡ��Aѡ�
���㣺���Ӽ���

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д������£����и���������ָ����Һ��һ���ܴ���������ǣ� ��
| A��0.1 mol��L��1��NaOH��Һ��K+��Na+��SO42����CO32�� |
| B��������Ӧ����������������Һ��Na+��K+��Cl����NO3�� |
| C��0.1 mol��L��1FeCl3��Һ��K+��NH4+��I����SCN�� |
| D��ʹ��ɫʯ����Һ������Һ��Ca2+��Na+��ClO����NO3�� |
�������ӷ���ʽ��ȷ����
A��������CO2ͨ�뱥��̼������Һ�У�CO2��CO32����H2O 2HCO3�� 2HCO3�� |
B��FeSO4��Һ�ڿ����б��ʣ�4Fe2+��O2��2H2O 4Fe3+��4OH�� 4Fe3+��4OH�� |
C����NaAlO2��Һ��ͨ�����CO2��2AlO2����CO2��3H2O 2Al(OH)3����CO32�� 2Al(OH)3����CO32�� |
D��̼����þ��Һ�м������ʯ��ˮ��Mg2++2HCO3��+2Ca2++4OH�� 2CaCO3��+ Mg(OH)2��+ 2H2O 2CaCO3��+ Mg(OH)2��+ 2H2O |
��pH=1����ɫ��Һ���ܴ����������������
| A��NH4+��Mg2+��SO42-��Cl- | B��Ba2+��K+��OH-��NO3- |
| C��Al3+��Cu2+��SO42-��Cl- | D��Na+��Ca2+��Cl-��AlO2- |
�������ӷ���ʽ��д��ȷ����
| A�����ڵ�NaHSO4����NaHSO4��Na + + H + + SO2�� 4 |
B��HCO�� 3��ˮ��Һ��ˮ�⣺ HCO�� 3��H2O H3O+ + CO2�� 3 H3O+ + CO2�� 3 |
| C��AlCl3��Һ�еμӹ�����ˮ�ķ�Ӧ�� Al3+ + 4OHһ�� A1O�� 2�� 2H2O |
| D�����Na2SO4��Һ��������Ӧ�� 2 H2O + 2eһ�� 2OHһ�� H2�� |
�������ӷ���ʽ��ȷ���� �� ��
| A������CO2ͨ�����ʯ��ˮ�У�CO2 + Ca2+ + 2OH��= CaCO3�� + H2O |
| B��Na2O2��ˮ������Ӧ��O22��+ H2O = 2OH��+ H2�� |
| C����AlCl3��Һ�м��������NaOH��Һ�� Al3+ + 3OH��= Al(OH)3�� |
| D��Ca(HCO3)2��������NaOH��Һ��Ӧ�� Ca2+ + 2HCO3��+ 2OH��= CaCO3��+ CO32��+ 2H2O |
�����£�������Һ�и�������һ�������������
| A��ʹ���ȳʺ�ɫ����Һ�У�Na+��AlO2����NO3����CO32�� |
| B��c(ClO��)��1.0 mol��L��1��Һ��Na+��SO32����S2����Cl�� |
| C������0.1 mol��L��1 HCO3-����Һ��Na����Fe3����NO3-��C6H5O�� |
D��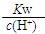 ��0.1 mol��L��1����Һ��Na����K����CO32-��NO3�� ��0.1 mol��L��1����Һ��Na����K����CO32-��NO3�� |
�������ӷ���ʽ��д��ȷ����
| A��ͭ��ϡ����ķ�Ӧ��Cu+4H++2NO3��= Cu2++2NO2��+2H2O |
B��������ˮ��Ӧ��Cl2+H2O H++Cl��+HClO H++Cl��+HClO |
| C������ϡ���ᷴӦ��2Fe+6H+=2Fe3++3H2�� |
| D�������Ȼ�����Һ��Ӧ��Fe+Fe3+=2Fe2+ |
���л�ѧ��Ӧ�����ӷ���ʽ��ȷ����( )
| A����ϡ������Һ�м�����3Fe+6H+=3Fe3++3H2�� |
| B��FeCl2��Һ��ͨ��Cl2��2Fe2++Cl2=2Fe3++2Cl- |
| C�����Ȼ�����Һ�м��������NaOH��Һ��Al3++3OH-=Al(OH)3�� |
D����ƫ��������Һ��ͨ�����CO2��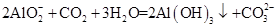 |