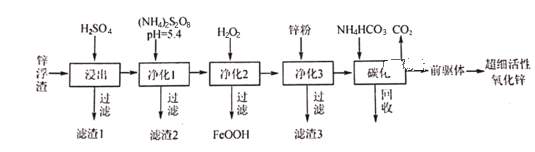
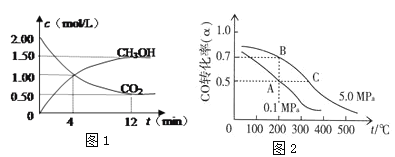
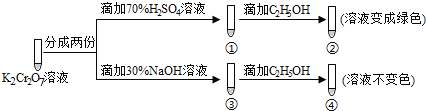
��Ŀ����
����Ŀ��25��ʱ����25mL0.1mol��L��1��NaOH��Һ�У���μ���0.2mol��L��1��CH3COOH��Һ����ҺpH�ı仯������ͼ��ʾ�����з����Ľ����У�����ȷ����

A.C��ʱc(CH3COO��)��c(Na��)��c(H��)��c(OH��)
B.D��ʱc(CH3COO��)��c(CH3COOH)��2c(Na��)
C.������A��B����һ�㣬��Һ�ж��У�c(Na��)��c(CH3COO��)>c(OH-)>c(H��)
D.B������a��12��5ml
���𰸡�D
��������
A�����ݵ���غ㣬c(CH3COO��)+c(OH��)=c(Na��)+c(H��)��C����Һ�����ԣ�����c(CH3COO��)��c(Na��)��c(H��)��c(OH��)����A��ȷ��
B��D��ʱc(CH3COO��)��c(CH3COOH)��2c(Na��)�����������غ㣬��B��ȷ��
C�������뼰��������ʱ��c(OH-)>c(CH3COO��)����C����
D��a��12.5mlʱ���������ƺʹ���ǡ����ȫ��Ӧ����ʱ��Һ�ʼ��ԣ���D����
��ѡD��

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�����Ŀ��Na2S������������Ⱦ�ϡ�����ˮ���е��ؽ����ȡ�
(1)Na2S��Һ��S2-ˮ������ӷ���ʽΪ_________��
(2)����ʱ�������ؽ������ӵ�������ܶȻ��������±���
�������� | FeS | PbS | CuS | HgS |
Ksp | 6.3��10��18 | 1.0��10��28 | 6.3��10��36 | 1.6��10��52 |
�������ʵ���Ũ����ͬ��Fe2+��Pb2+��Cu2+��Hg2+�Ļ��ϡ��Һ�У���μ���Na2Sϡ��Һ�����ȳ�����������____��
����Na2S��Һ������ˮ��Pb2+��ΪʹPb2+������ȫ[c(Pb2��)��1��10-6mol/L]����Ӧ������Һ��c(S2-)��_____mol/L��
�۷�ӦCu2+(aq)+FeS(s)![]() Fe2+(aq)+CuS(s)��ƽ�ⳣ��K=_______��
Fe2+(aq)+CuS(s)��ƽ�ⳣ��K=_______��
(3)�ⶨijNa2S��NaHS�����Ʒ�����ߺ�����ʵ�鲽�����£�
����1.ȷ��ȡһ������Ʒ���ձ��У�������������ˮ�ܽ⣬ת����500mL����ƿ�ж��ݡ�
����2.ȷ��ȡ25.00mL������Һ����ƿ�У��������ػ�GG-��ʱ��������ָʾ������0.2500mol/L�������Һ�ζ���Na2S+HCl=NaHS+NaCl�����յ㣬��������24.00mL���������ټ���5mL���Լ�ȩ(NaHS+HCHO+H2O��NaOH+HSCH2OH)��3�η�ָ̪ʾ����������0.2500mol/L�������Һ�ζ�(NaOH+HCl=NaCl+H2O)���յ㣬����������34.00mL��
����ԭ�������Na2S��NaHS�����ʵ���֮��(д���������)___________��