��Ŀ����
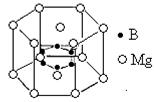
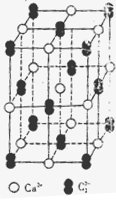
2004��7�µ¶�������ѧ�ҹ�ͬ�������ڸ�ѹ�µ����ᷢ���ۺϵõ��߾۵������ָ߾۵���N��N���ļ���Ϊ160kJ/ mol��N2�����еļ���Ϊ942kJ/mol��������ṹ��ͼ��ʾ�������йظ߾۵���˵������ȷ����
mol��N2�����еļ���Ϊ942kJ/mol��������ṹ��ͼ��ʾ�������йظ߾۵���˵������ȷ����
 mol��N2�����еļ���Ϊ942kJ/mol��������ṹ��ͼ��ʾ�������йظ߾۵���˵������ȷ����
mol��N2�����еļ���Ϊ942kJ/mol��������ṹ��ͼ��ʾ�������йظ߾۵���˵������ȷ����
| A���߾۵��������ڷ��Ӿ��� |
| B���߾۵�������ÿ��Nԭ�Ӻ�����3��Nԭ������ |
| C���߾۵�ת��ɵ�����������ԭ��Ӧ |
| D���߾۵����ܳ�Ϊըҩ |
AC
��

��ϰ��ϵ�д�
�����Ŀ

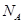 �ı���ʽΪ ��
�ı���ʽΪ ��
 �γ���λ��Ϊ6���������к��ѵ�������ɫ�ľ��壬һ��Ϊ��ɫ����һ��Ϊ��ɫ�������ʵ��֤�������־������ɽ�ΪTiCl3��6H2O��Ϊ�ⶨ�����־���Ļ�ѧʽ�����������ʵ�飺a���ֱ�ȡ����������������ᄃ�����Ʒ��ɴ�����Һ��b���ֱ�������
�γ���λ��Ϊ6���������к��ѵ�������ɫ�ľ��壬һ��Ϊ��ɫ����һ��Ϊ��ɫ�������ʵ��֤�������־������ɽ�ΪTiCl3��6H2O��Ϊ�ⶨ�����־���Ļ�ѧʽ�����������ʵ�飺a���ֱ�ȡ����������������ᄃ�����Ʒ��ɴ�����Һ��b���ֱ������� ��Һ�е���AgNO3��Һ����������ɫ������c��������ȫ��ֱ���˵����ݳ�������ϴ�Ӹ�������������ԭ��ɫ�����ˮ��Һ��AgNO3��Һ��Ӧ�õ��İ�ɫ��������Ϊ��ɫ�����ˮ��Һ��Ӧ�õ�����������2/3������ɫ���������Ļ�ѧʽΪ ��
��Һ�е���AgNO3��Һ����������ɫ������c��������ȫ��ֱ���˵����ݳ�������ϴ�Ӹ�������������ԭ��ɫ�����ˮ��Һ��AgNO3��Һ��Ӧ�õ��İ�ɫ��������Ϊ��ɫ�����ˮ��Һ��Ӧ�õ�����������2/3������ɫ���������Ļ�ѧʽΪ ��