��Ŀ����
��16�֣��۲����в���ͼ�Σ�����Ҫ��ش���������

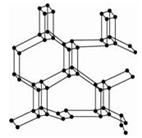
(1)�ɽ��ʯ����ṹ�;���ͼ֪�����ʯ��ԭ�Ӿ��壬������̼ԭ��ȡ
�ӻ�����γɦҼ���ÿ�������к�̼ԭ����Ϊ ����
(2)�����ӣ�P4���м���Ϊ �����ӵĿռ�ṹΪ ��ÿ1mol�����Ӻ� mol P-P���ۼ�������1���Ӱ���������P-P����������һ����ԭ������������ķ���ʽΪ ����ÿ��Pԭ�ӵŶԵ���������ԭ����λ���Ϳɵõ�����һ�������� �������ʽ����
(3)��SiO2����ṹ֪SiO2������ ���壬ÿ1mol SiO2���庬 mol Si-O���ۼ���
(4)��֪CaC2����ľ����ṹ��NaCl�������ƣ�����ͼ����CaC2������������C22-�Ĵ��ڣ�ʹ������һ��������������CaC2������Ca2+��λ����C.N��Ϊ ��C22-��O22+��Ϊ�ȵ����壬O22+�ĵ���ʽΪ ��1molO22+�к��м���ĿΪ ��
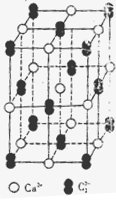
(5)������Ļ����ṹ��Ԫ��������ԭ����ɵ�����ʮ�����ԭ�Ӿ��塣���к���20���ȱ������κ�һ����Ŀ�Ķ��ǣ�ÿ�����Ǹ���һ��ԭ�ӣ��۲�ͼ�λش���������ṹ��Ԫ�� ����ԭ����ɣ������� ��B-B����
(6)�����mg NaCl��������ΪVcm3,��֪NaCl�����У����������Na+��Cl-��ľ���Ϊa cm,��ӵ�����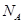 �ı���ʽΪ ��
�ı���ʽΪ ��

(1)�ɽ��ʯ����ṹ�;���ͼ֪�����ʯ��ԭ�Ӿ��壬������̼ԭ��ȡ
�ӻ�����γɦҼ���ÿ�������к�̼ԭ����Ϊ ����
(2)�����ӣ�P4���м���Ϊ �����ӵĿռ�ṹΪ ��ÿ1mol�����Ӻ� mol P-P���ۼ�������1���Ӱ���������P-P����������һ����ԭ������������ķ���ʽΪ ����ÿ��Pԭ�ӵŶԵ���������ԭ����λ���Ϳɵõ�����һ�������� �������ʽ����
(3)��SiO2����ṹ֪SiO2������ ���壬ÿ1mol SiO2���庬 mol Si-O���ۼ���
(4)��֪CaC2����ľ����ṹ��NaCl�������ƣ�����ͼ����CaC2������������C22-�Ĵ��ڣ�ʹ������һ��������������CaC2������Ca2+��λ����C.N��Ϊ ��C22-��O22+��Ϊ�ȵ����壬O22+�ĵ���ʽΪ ��1molO22+�к��м���ĿΪ ��

(5)������Ļ����ṹ��Ԫ��������ԭ����ɵ�����ʮ�����ԭ�Ӿ��塣���к���20���ȱ������κ�һ����Ŀ�Ķ��ǣ�ÿ�����Ǹ���һ��ԭ�ӣ��۲�ͼ�λش���������ṹ��Ԫ�� ����ԭ����ɣ������� ��B-B����
(6)�����mg NaCl��������ΪVcm3,��֪NaCl�����У����������Na+��Cl-��ľ���Ϊa cm,��ӵ�����
 �ı���ʽΪ ��
�ı���ʽΪ ����16�֣���1��SP3, 8 ��
��2��60��, ����������6 �� P4O6, P4O10 .
��3��ԭ���� 4��
��4�� 4 ��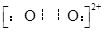 �� 2NA�� ��
�� 2NA�� ��
��5�� 12 �� 30 ��
��6�� ��������6��2�֣�����ÿ��1�֣�
��������6��2�֣�����ÿ��1�֣�
��2��60��, ����������6 �� P4O6, P4O10 .
��3��ԭ���� 4��
��4�� 4 ��
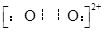 �� 2NA�� ��
�� 2NA�� ����5�� 12 �� 30 ��
��6��
 ��������6��2�֣�����ÿ��1�֣�
��������6��2�֣�����ÿ��1�֣���

��ϰ��ϵ�д�
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
�����Ŀ


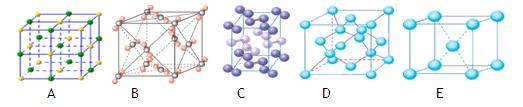
 ��
�� mol��N2�����еļ���Ϊ942kJ/mol��������ṹ��ͼ��ʾ�������йظ߾۵���˵������ȷ����
mol��N2�����еļ���Ϊ942kJ/mol��������ṹ��ͼ��ʾ�������йظ߾۵���˵������ȷ����