��Ŀ����
��1�������£���20.0 g 14%��NaCl��Һ��30.0 g 24%��NaCl��Һ��ϣ��õ��ܶ�Ϊ1.17 g/cm3�Ļ����Һ,�û����Һ��NaCl�����ʵ���Ũ��Ϊ mol/L��
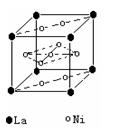
��2�����������������Դ���磨La��������Ni���ĺϽ����������ϡ���ͼΪ�úϽ�ľ���ṹ����С���ظ��ṹ��Ԫ����������һ����ԭ�ӣ�������ԭ�Ӷ������ϣ���ԭ�Ӷ��ڶ����ϡ��þ���Ļ�ѧʽΪ ��
��3��������ˮ����ˮ����Ի�����ɵ���ȾԽ��Խ���أ�����ר����Ϊ�����ý���þ��ˮ���е�NO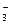 ��ԭΪN2���Ӷ�������Ⱦ��
��ԭΪN2���Ӷ�������Ⱦ��
��д��þ�ͺ�����ˮ��Ӧ�����ӷ���ʽ ��
��������Ӧ�У����ɱ�״����33��6L����ʱ��ת�Ƶ��ӵ����ʵ���Ϊ mol������֪����þ���ԴӺ�ˮ����ȡ��MgCI2ͨ������Ƶõģ���Ҫ��ȥ����Ԫ��0.3mol�ķ�ˮ�е�NO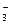 ����������Ҫ��0.5%������������MgCl2�ĺ�ˮ kg��
����������Ҫ��0.5%������������MgCl2�ĺ�ˮ kg��

��2�����������������Դ���磨La��������Ni���ĺϽ����������ϡ���ͼΪ�úϽ�ľ���ṹ����С���ظ��ṹ��Ԫ����������һ����ԭ�ӣ�������ԭ�Ӷ������ϣ���ԭ�Ӷ��ڶ����ϡ��þ���Ļ�ѧʽΪ ��
��3��������ˮ����ˮ����Ի�����ɵ���ȾԽ��Խ���أ�����ר����Ϊ�����ý���þ��ˮ���е�NO
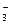 ��ԭΪN2���Ӷ�������Ⱦ��
��ԭΪN2���Ӷ�������Ⱦ����д��þ�ͺ�����ˮ��Ӧ�����ӷ���ʽ ��
��������Ӧ�У����ɱ�״����33��6L����ʱ��ת�Ƶ��ӵ����ʵ���Ϊ mol������֪����þ���ԴӺ�ˮ����ȡ��MgCI2ͨ������Ƶõģ���Ҫ��ȥ����Ԫ��0.3mol�ķ�ˮ�е�NO
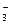 ����������Ҫ��0.5%������������MgCl2�ĺ�ˮ kg��
����������Ҫ��0.5%������������MgCl2�ĺ�ˮ kg����1��4.0
��2�� LaNi5��Ni5La
��3����2NO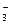 +5Mg+6H2O =N2��+5Mg��OH��2+2OH-
+5Mg+6H2O =N2��+5Mg��OH��2+2OH-
��15
��14��25
��2�� LaNi5��Ni5La
��3����2NO
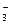 +5Mg+6H2O =N2��+5Mg��OH��2+2OH-
+5Mg+6H2O =N2��+5Mg��OH��2+2OH- ��15
��14��25
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
