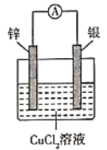
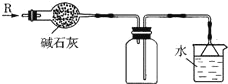
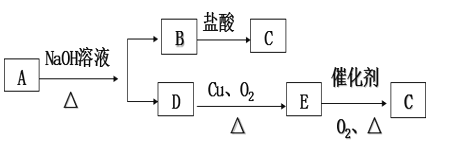
��Ŀ����
����Ŀ����������һ����Ҫ�Ļ����м��壬������ҵ�о����ȵ㡣һ����ʯ�ͻ����еķ�����������Ҫ�ɷ�ΪNiCO3��SiO2������������Fe2O3��Cr2O3��Ϊԭ���Ʊ��������Ĺ�ҵ������ͼ��

��֪����NiS��Ni(OH)2��Cr(OH)3��������ˮ��Cr(OH)3�������������
��Fe(OH)3������NH4Cl-��ˮ�Ļ��Һ��Ni(OH)2����NH4Cl-��ˮ�Ļ��Һ����[Ni(NH3)6]2+��
��ش��������⣺
��1�������ܡ�ʱӦ�Ƚ������������飬����20%������100���·�Ӧ2h���ò�����Ŀ��Ϊ____��
��2������������Ҫ�ɷ�Ϊ___���ѧʽ�����������ڹ�ҵ�ϵ���;Ϊ___����дһ�֣���
��3����һ�μ�����ʱ�������NaOH��Һ�������������������Ӧ�����ӷ���ʽΪ__��
��4�������⡱��Ŀ��Ϊ___����������ʱͨ��H2S��Ŀ����___��
��5����������ʱ������Ӧ�����ӷ���ʽΪ___��
��6����ϵ�в�����������ָ____�����ˡ�ϴ�ӡ����������NiSO4��7H2O���岻����Ӧ�������е��ᴿ��������Ϊ____��
���𰸡��ӿ췴Ӧ���ʣ������Ԫ�صĽ����� SiO2 �Ʋ��������ƹ��ά�� Cr3++4OH-=CrO2-+2H2O ʵ����Ԫ�غ���Ԫ�صķ��� ����Ԫ��ת��ΪNiS���� 3NiS+8H++2NO3-=3Ni2++2NO��+3S+4H2O ����Ũ������ȴ�ᾧ �ؽᾧ
��������
��1���������������飬�������������������³�ʱ�䷴Ӧ��Ŀ���Ǽӿ췴Ӧ���ʣ������Ԫ�صĽ����ʡ�
��2��NiCO3��Fe2O3��Cr2O3���������ᣬSiO2���������ᣬ��������������Ҫ�ɷ�ΪSiO2����ҵ�Ͽ�����SiO2�Ʋ������ƴֹ衢�ƹ��ά�ȡ�
��3��Cr(OH)3�������������NaOH��Һ������Cr3+ת��ΪCrO2-��
��4��Fe(OH)3������NH4Cl-��ˮ�Ļ��Һ��Ni(OH)2����NH4Cl-��ˮ�Ļ��Һ����[Ni(NH3)6]2+��������������Ŀ��Ϊʵ����Ԫ�غ���Ԫ�صķ��롣��������ʱͨ��H2S��Ŀ���ǽ���Ԫ��ת��ΪNiS������
��5����������ʱHNO3����ԭΪNO��NiS������ΪS����Ӧ�����ӷ���ʽΪ3NiS+8H++2NO3-=3Ni2++2NO��+3S+4H2O��
��6����NiSO4��Һ�л��NiSO4��7H2O�IJ���Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����������NiSO4��7H2O���岻����Ӧ���������ؽᾧ���ᴿ��