��Ŀ����
12��ij��A����Է�������Ϊ84���ش��������⣺��1������������A�����������ϣ��������ʵ���һ�������ȼ������������������ȵ��ǣ�����ţ�b��a��C7H12O2 b��C6H14 c��C6H14O d��C7H14O3
��2������AΪ��������HBr�ӳɺ�ֻ�ܵõ�һ�ֲ���Ҹ�����һ�ȴ���ֻ��һ�֣�
��A�Ľṹ��ʽΪ
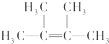 ��������2��3-����-2-��ϩ��
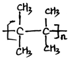
��������2��3-����-2-��ϩ����A��һ���������ܷ����Ӿ۷�Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽn��CH3��2C=C��CH3��2$\stackrel{һ������}{��}$
 ��
����A����������ˮ��Ӧ����B��B��NaOH�Ĵ���Һ���ȿ��Եõ�D��B��D����Է�����������M��D��+81=M��B������D�����к��еĹ������У���ԭ�Ӻ�̼̼˫���������ƣ���
��3�����˴Ź���������ʾ����A���������壬�ҷ������Ϊ3��2��1��д��A���п��ܵĽṹ��ʽ
 ����CH3CH2��2C=CH2��
����CH3CH2��2C=CH2��
���� ��A����Է�������Ϊ84�����������Ϊϩ��������������ʽӦΪC6H12��
��1�������ʵ���һ�������ȼ������������������ȣ����CxHyOz��������Ϊx+$\frac{y}{4}$-$\frac{z}{2}$��������
��2����AΪ��������HBr�ӳɺ�ֻ�ܵõ�һ�ֲ���Ҹ�����һ�ȴ���ֻ��һ�֣���ṹ�Գƣ�ֻ��һ��H������AΪ��CH3��2C=C��CH3��2��A����������ˮ�����ӳɷ�Ӧ����BΪ��CH3��2CBrCBr��CH3��2��B��NaOH�Ĵ���Һ���ȿ��Եõ�D��B��D����Է�����������M��D��+81=M��B������B��ȥһ��HBr���ӵ�DΪ��CH3��2CBrC��CH3��=CH2���ݴ˴��⣻
��3���˴Ź���������ʾ����A�����鲻ͬ�ķ壬�������Ϊ3��2��1����AΪCH2=C��CH2CH3��CH2CH3��
��� �⣺��A����Է�������Ϊ84�����������Ϊϩ��������������ʽӦΪC6H12��
��1�������ʵ���һ�������ȼ������������������ȣ���1molC6H12��������Ϊx+$\frac{y}{4}$=9mol��
a��1molC7H12O2��������Ϊ9mol�������ʵ���һ�������ȼ����������������ȣ���a��ѡ��
b��1molC6H14��������Ϊ9.5mol�������ʵ���һ�������ȼ������������������ȣ���bѡ��
c��1molC6H14O��������Ϊ9mol�������ʵ���һ�������ȼ����������������ȣ���c��ѡ��
d��1molC7H14O3��������Ϊ9mol�������ʵ���һ�������ȼ����������������ȣ���d��ѡ��
�ʴ�Ϊ��b��
��2����AΪ��������HBr�ӳɺ�ֻ�ܵõ�һ�ֲ���Ҹ�����һ�ȴ���ֻ��һ�֣���ṹ�Գƣ�ֻ��һ��H������AΪ��CH3��2C=C��CH3��2��A����������ˮ�����ӳɷ�Ӧ����BΪ��CH3��2CBrCBr��CH3��2��B��NaOH�Ĵ���Һ���ȿ��Եõ�D��B��D����Է�����������M��D��+81=M��B������B��ȥһ��HBr���ӵ�DΪ��CH3��2CBrC��CH3��=CH2��
��������ķ�����֪��AΪ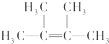 ������Ϊ2��3-����-2-��ϩ��
������Ϊ2��3-����-2-��ϩ��
�ʴ�Ϊ��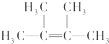 ��2��3-����-2-��ϩ��
��2��3-����-2-��ϩ��
��A��һ���������ܷ����Ӿ۷�Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪn��CH3��2C=C��CH3��2 $\stackrel{һ������}{��}$  ��
��
�ʴ�Ϊ��n��CH3��2C=C��CH3��2 $\stackrel{һ������}{��}$  ��
��
��������ķ�����֪��DΪ��CH3��2CBrC��CH3��=CH2��D�к��еĹ�����Ϊ��ԭ�Ӻ�̼̼˫����
�ʴ�Ϊ����ԭ�Ӻ�̼̼˫����
��3���˴Ź���������ʾ����A�����鲻ͬ�ķ壬�������Ϊ3��2��1����AΪ ����CH3CH2��2C=CH2��
����CH3CH2��2C=CH2��
�ʴ�Ϊ�� ����CH3CH2��2C=CH2��
����CH3CH2��2C=CH2��
���� ���⿼���л�����ƶϣ���ȷ��Է��������Ƴ�AΪϩ���������ǽ���ͻ�ƿڣ�ע�������л���ṹ�����ʵĹ�ϵ�������Ŀ�Ѷ��еȣ�

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�| A�� | ���ʷ�����ѧ��Ӧ�������������仯 | |
| B�� | ���ȷ�Ӧһ����Ҫ���Ȳ��ܷ��� | |
| C�� | ���Ƿ��ȷ�Ӧ�ķ������������ | |
| D�� | ���������仯�����ʱ仯���ǻ�ѧ�仯 |
| A�� | H2��CO2 | B�� | CO2��H2O | C�� | CO��CH3OH | D�� | CH3OH��H2 |
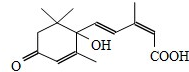 �����ڼ�Դ��������ʻ�ʩ��S-�տ����Ƽ����Ա�֤�ʻ�ʢ����S-�տ��صķ��ӽṹ��ͼ������˵����ȷ���ǣ�������
�����ڼ�Դ��������ʻ�ʩ��S-�տ����Ƽ����Ա�֤�ʻ�ʢ����S-�տ��صķ��ӽṹ��ͼ������˵����ȷ���ǣ�������| A�� | 1mol�÷��ӿ���1molNa������Ӧ����H2 | |
| B�� | �����к������ֹ����� | |
| C�� | �ɷ����ӳɷ�Ӧ��ȡ����Ӧ | |
| D�� | �÷��Ӳ���ʹ���Ը��������Һ��ɫ |
��1��������Ӧ��ƽ�ⳣ������ʽK=$\frac{{c}^{4}��{H}_{2}��}{{c}^{4}��{H}_{2}O��}$��
��2����֪����3Fe��s��+2O2��g���TFe3O4��s����H1=-1118.4kJ•mol-1��
��2H2��g��+O2��g���T2H2O��g����H2=-483.8kJ•mol-1��
��2H2��g��+O2��g���T2H2O��l����H3=-571.8kJ•mol-1��
���H=-150.8kJ/mol
��3����֪��t��ʱ���÷�Ӧ��ƽ�ⳣ��K=16����2L���º����ܱ����������У��ֱ������ʾ�������ʣ�����һ��ʱ���ﵽƽ�⣮
| Fe | H2O��g�� | Fe3O4 | H2 | |
| ��/mol | 1.0 | 1.0 | 1.0 | 1.0 |
| ��/mol | 1.0 | 1.5 | 1.0 | 1.0 |
������˵������ȷ����B�����ţ���
A��������ѹǿ�㶨����Ӧ�ﵽƽ��״̬
B���������������ܶȺ㶨����Ӧ�ﵽƽ��״̬
C����������H2O��ƽ��ת���ʴ�����������H2O��ƽ��ת����
D������Fe3O4�������H2O��ת����
��4��������2L���ݾ��ȣ�������罻����������װ���У������������ʼ���ʣ���ʼʱ��ƽ���ĸ����ʵ���������
| Fe | H2O��g�� | Fe3O4 | H2 | |
| ��ʼ/mol | 3.0 | 4.0 | 0 | 0 |
| ƽ��/mol | m | n | p | q |
| Fe | H2O��g�� | Fe3O4 | H2 | |
| A/mol | 3.0 | 4.0 | 0 | 0 |
| B/mol | 0 | 0 | 1.0 | 4.0 |
| C/mol | m | n | p | q |
��5����֪������Fe��OH��3��KSP=4.0��10-39����ijFeCl3��Һ��pH��Ϊ3����ʱ��Һ��c��Fe3+��=4.0��10-6mol•L-1���������2λ��Ч���֣���
 B��
B�� C��
C��
 ��
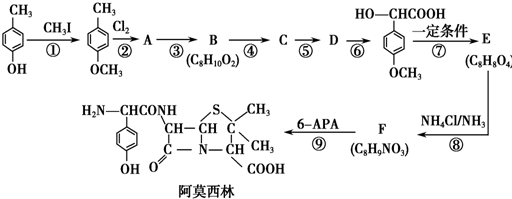
��
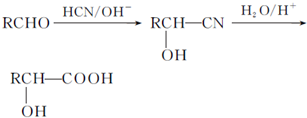

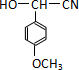 ��
��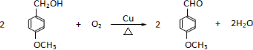 ��
��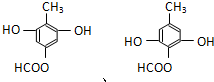 ��
��