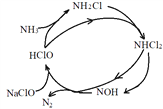
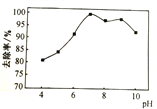
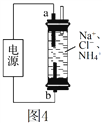
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ“ΜΕ®Έ¬Ε»œ¬Θ§»ΐ÷÷ΧΦΥα―ΈMCO3(MΘΚMg2ΘΪΓΔCa2ΘΪΓΔMnΘΪ)ΒΡ≥ΝΒμ»ήΫβΤΫΚβ«ζœΏ»γΆΦΥυ ΨΓΘœ¬Ν–ΥΒΖ®¥μΈσΒΡ «Θ® Θ©

A. Ksp(MnCO3)ΒΡ ΐΝΩΦΕΈΣ10Θ≠11
B. MgCO3ΒΡ–ϋΉ«“Κ÷–Φ”»κ…ΌΝΩΥ°≥δΖ÷’ώΒ¥Θ§c(Mg2ΘΪ)≤Μ±δ
C. œρ≈®Ε»ΨυΈΣ0.01molΓΛLΘ≠1ΒΡMg2ΘΪΓΔCa2ΘΪΓΔMn2ΘΪΜλΚœ»ή“Κ÷–÷πΒΈΦ”»κNa2CO3»ή“ΚΘ§Ήνœ»–Έ≥…MgCO3≥ΝΒμ
D. aΒψ±μ ΨΒΡ»ή“Κ÷–Θ§c(Ca2ΘΪ)ΘΨc(CO32Θ≠)
ΓΨ¥πΑΗΓΩC
ΓΨΫβΈωΓΩ
A.ΗυΨίΤπΒψΘΚ-lgc(Mn2+)Γ÷11, -lgc(CO32-)=0Θ§Ω…ΒΟ Ksp(MnCO3)ΒΡ ΐΝΩΦΕΈΣ10Θ≠11Θ§Ι ≤Μ―ΓAΘΜ
B. MgCO3ΒΡ–ϋΉ«“Κ÷–Φ”»κ…ΌΝΩΥ°≥δΖ÷’ώΒ¥Θ§»‘»Μ «±ΞΚΆ»ή“ΚΘ§Έ¬Ε»≤Μ±δΘ§Ksp≤Μ±δΘ§c(Mg2ΘΪ)≤Μ±δΘ§Ι ≤Μ―ΓBΘΜ
C.œρ≈®Ε»ΨυΈΣ0.01molΓΛLΘ≠1ΒΡMg2ΘΪΓΔCa2ΘΪΓΔMn2ΘΪΜλΚœ»ή“Κ÷–÷πΒΈΦ”»κNa2CO3»ή“ΚΘ§KspΉν–ΓΒΡœ»¥οΒΫ±ΞΚΆΘ§…ζ≥…≥ΝΒμΘ§ΗυΨίΆΦœώΩ…÷ΣKspΘ®MnCO3Θ©<KspΘ®CaCO3Θ©< KspΘ®MgCO3Θ©Θ§“ρ¥ΥΉνœ»–Έ≥…MnCO3≥ΝΒμΘ§Ι ―ΓCΘΜ
D.“ρΈΣΚαΉίΉχ±ξΨυΈΣΗΚΕ‘ ΐΘ§÷Β‘Ϋ¥σΘ§άκΉ”≈®Ε»‘Ϋ–ΓΘ§Υυ“‘aΒψ±μ ΨΒΡ»ή“Κ÷–Θ§c(Ca2ΘΪ)ΘΨc(CO32Θ≠)Θ§Ι ≤Μ―ΓDΘΜ
¥πΑΗΘΚC

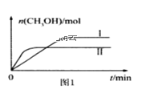
 ΝΝΒψΦΛΜνΨΪ±ύΧα”≈100Ζ÷¥σ ‘ΨμœΒΝ–¥πΑΗ
ΝΝΒψΦΛΜνΨΪ±ύΧα”≈100Ζ÷¥σ ‘ΨμœΒΝ–¥πΑΗΓΨΧβΡΩΓΩνή‘ΣΥΊ”…”ΎΤδΝΦΚΟΒΡΈοάμΜ·―ß–‘÷ Θ§±ΜΙψΖΚ”Π”Ο”Ύ…ζ≤ζ…ζΜν÷–ΓΘ¥”Κ§νήΖœΝœ(Κ§CoOΓΔCo2O3ΓΔΒΞ÷ AlΓΔLiΒ»)÷–÷Τ»Γ¥÷CoCl2ΓΛ6H2OΒΡΝς≥Χ»γœ¬Υυ ΨΓΘ

«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)≤Ϋ÷ηI÷–÷ς“ΣΖΔ…ζΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΈΣ______ΓΘ
(2)“―÷ΣCo2O3ΨΏ”–«Ω―θΜ·–‘Θ§»τ≤Ϋ÷ηII÷–Ϋΰ≥ωΦΝΈΣ―ΈΥαΘ§‘λ≥…ΒΡΚσΙϊ «_______ΓΘ
(3)≤Ϋ÷ηΔσ÷–ΔΌΒΡΡΩΒΡ «≥ΐ»ΞAl3+Θ§–¥≥ωΗΟ≤ΫΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ______ΓΘ
(4)»τ‘Ύ Β―ι “λ―…’CoCO3Θ§Υυ–ηΒΡΙηΥα―Έ÷ “«Τς≥ΐΨΤΨΪΒΤΚΆ≤ΘΝßΑτΆβΘ§ΜΙ”–______ΓΔ______(Χν“«ΤςΟϊ≥Τ)ΓΘ
(5)≤ΌΉςΔΌ «‘ΎHClΖ’Έß÷–Ϋχ––ΒΡΘ§Τδ≤Ϋ÷η «______ΓΔ_____ΓΔΙΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΓΘœ¥Β”Ιΐ≥Χ÷–Ω…“‘”ΟΙΛ“ΒΨΤΨΪ¥ζΧφΥ°Θ§Τδ”≈Βψ «_____ΓΘ
(6)Ρ≥Ά§―ß”Ο±ξΉΦœθΥα“χ»ή“ΚΒΈΕ®Έ¥÷Σ≈®Ε»ΒΡCoCl2»ή“ΚΘ§œ¬Ν–Ω…ΉςΈΣ÷Η ΨΦΝΒΡ «____(Χν―ΓœνΘ§Κω¬‘―«νήάκΉ”ΒΡ―’…ΪΗ…»≈)
AΘ°KCl BΘ°KSCN CΘ°K2CrO4 DΘ°K2S
“―÷ΣΦΗ÷÷Έο÷ ‘Ύ20Γφ ±ΒΡ―’…ΪΦΑKsp÷Β»γœ¬±μ
Μ·―ß Ϋ | AgCl | AgSCN | Ag2S | Ag2CrO4 |
―’…Ϊ | ΑΉ…Ϊ | «≥ΜΤ…Ϊ | ΚΎ…Ϊ | Κλ…Ϊ |
Ksp | 2.0ΓΝ10-10 | 1.0ΓΝ10-12 | 2.0ΓΝ10-48 | 2.0ΓΝ10-12 |