��Ŀ����
��ѧ������ѧ��Ӧ����ʽΪ��A+B��X+Y+H2O��δ��ƽ����Ӧ������ȥ�������У�A��B�����ʵ���֮��Ϊ1:4����ش�
��1����YΪ����ɫ���壬�÷�Ӧ�����ӷ���ʽΪ ��B���ֳ��Ļ�ѧ������
��2����AΪ�����ķǽ������ʣ�B����ҺΪijŨ�ᣬ��Ӧ����Ϊ���ȣ��䷴Ӧ�Ļ�ѧ����ʽΪ
��3����AΪij�����õĽ������ʣ�ʵ���ҳ��ø÷�Ӧ���Ʊ�ij�����γ���������壬�÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ
��4����AΪ�����Ľ������ʣ�������A��B��Ũ��Һ�С��ۻ�������A������X��Һ�С�
��AԪ����Ԫ�����ڱ��е�λ����
�ں�amolX����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X�����ʵ�����
��1��MnO2+4H++2Cl�� Mn2++2H2O+Cl2�� ���Ժͻ�ԭ��
Mn2++2H2O+Cl2�� ���Ժͻ�ԭ��
��2��C+4HNO3(Ũ��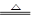 CO2��+4NO2��+2H2O
CO2��+4NO2��+2H2O
��3���漰��ӦΪ��Cu��4HNO3(Ũ)��Cu(NO3)2��2NO2����2H2O ���������뻹ԭ�������ʵ���֮��Ϊ 2:1
��4���ٵ������ڵڢ��� ��0��4a ����2�֡�
���������������1������ɫ����һ��Ϊ��������˷�ӦΪ��MnO2+4H++2Cl��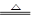 Mn2++2H2O+Cl2�� ���������Ӧ��������ֳ���������Ϊ�����Ժͻ�ԭ�ԡ�
Mn2++2H2O+Cl2�� ���������Ӧ��������ֳ���������Ϊ�����Ժͻ�ԭ�ԡ�
��2��C+4HNO3(Ũ��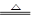 CO2��+4NO2��+2H2O
CO2��+4NO2��+2H2O
��3��2:1
��4���������֪������Ԫ��ΪFe���������ڱ��е�λ��Ϊ���������ڵڢ���
�� Fe �� 2Fe3�� �� 3Fe2��
��ʼ��Xmol 2amol 0
��Ӧ��bmol 2bmol 3bmol
���գ���x-b��mol (2a-2b)mol 3bmol
��������ṩ������(2a-2b)= 3b������b=0��4a
��˱���ԭ��Fe3�������ʵ���Ϊ0��8a����ô����ԭ��XΪ0��4amol��
���㣺��������֮���ת����

������ͼ��ʾװ����ȡ����Ҫʱ�ɼ��ȣ����������ռ��������� 
| A��ͭ��ϡ���ᷴӦ��һ������ |
| B���Ȼ�����������ƹ��巴Ӧ�ư��� |
| C��п��ϡ���ᷴӦ������ |
| D���������ƹ��������ᷴӦ�ƶ������� |