��Ŀ����
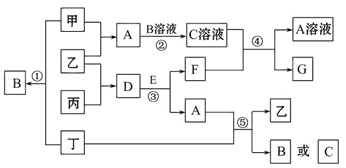
��ͼ��ʾ������ת����ϵ�У������ʾ�Ϊ����������Ԫ����ɵĵ��ʻ����֪��A��C��D��F��K��Ϊ���ʣ�C��E��F��G��K�����������壬��KΪ��ҵ������Ư�۵�ԭ��֮һ��JΪ��ɫ�����Ҽ�������B��ˮ��Һ����������E��ˮ��Һ��B��G����ʹʪ��ĺ�ɫʯ����ֽ��������Ӧ���ǹ�ҵ�ƻ��ʵ���Ҫ��Ӧ֮һ����ͼ�в��ַ�Ӧ����������δ�г���
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2��H�Ļ�ѧʽΪ ��
��3��д����Ӧ�ܵĻ�ѧ����ʽ ��
��4��д����Ӧ�ݵ����ӷ���ʽ ��
��1��
��2�� NaAlO2
��3��N2+3H2 2NH3
2NH3
��4��Al3++3NH3+3H2O=Al(OH)3��+3NH4+ �� Al3++3NH3��H2O=Al(OH)3��+3NH4+
�������������KΪ��ҵ������Ư�۵�ԭ��֮һ����֪kΪ������B��G����ʹʪ��ĺ�ɫʯ����ֽ������˵��ˮ��Һ�ʼ��ԣ�GΪ������CΪ������FΪ������JΪ��ɫ�����Ҽ�������B��ˮ��Һ����������E��ˮ��Һ��˵��jΪ����������AΪ�ƣ�BΪ�������ơ�DΪ����HΪƫ�����ƣ�IΪ�Ȼ������� B�ĵ���ʽΪ ���� H�Ļ�ѧʽΪ NaAlO2��
���� H�Ļ�ѧʽΪ NaAlO2��
�� д����Ӧ�ܵĻ�ѧ����ʽN2+3H2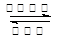 2NH3 ���� д����Ӧ�ݵ����ӷ���ʽAl3++3NH3+3H2O=Al(OH)3��+3NH4+ �� Al3++3NH3��H2O=Al(OH)3��+3NH4+��
2NH3 ���� д����Ӧ�ݵ����ӷ���ʽAl3++3NH3+3H2O=Al(OH)3��+3NH4+ �� Al3++3NH3��H2O=Al(OH)3��+3NH4+��
���㣺���⿼��������ͼ�����ʵ��ƶϡ�


 Mn2����2H2O��Cl2��
Mn2����2H2O��Cl2��