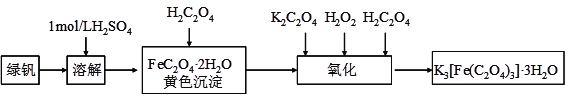��Ŀ����
����Ŀ����������ӵ�ص������ζ������������[LiBF2(C2O4)]�ĺϳɷ������£�2H2C2O4+SiCl4+2LiBF4��2LiBF2(C2O4)+SiF4+4HCl
��1������ͬ��ĵ�������Ԫ��ԭ�ӵĻ�̬��������Ų�ʽΪ_______________________��
��2���������(�Ҷ��ᣬH2C2O4)��̼ԭ�ӹ�����ӻ�������_____________��1mol��������к��ЦҼ�����ĿΪ____________��
��3����SiF4��Ϊ�ȵ�����������ӵĻ�ѧʽΪ__________��BF4-�Ŀռ乹��Ϊ_____________��
��4����һ�����ܽ���B��F����Ԫ��֮��ĵڶ�����Ԫ����__________�֡�
��5����֪NixO���徧���ṹΪ�Ȼ����ͣ����ھ���ȱ�ݣ�xֵС��1��һ��Ni2+��ȱ����������Ni2+������Ni3+ȡ����ʹ��������Ni��O�ı�ֵ�����˱仯��ij��������Ʒ���ΪNi0.93O���˾��廯ѧʽΪ_______________________�����ú�Ni2+��Ni3+��ʾ��
���𰸡� [Ar]3d104s24p5 sp2�ӻ� 7mol SO42-��PO43-�� ���������� 4 Ni2+0.79Ni3+0.14O
����������1������ͬ��ĵ�������Ԫ��Br��35��Ԫ�أ�ԭ�ӵĻ�̬��������Ų�ʽΪ[Ar]3d104s24p5��1s22s22p63s23p63d104s24p5����4�����ᣨH2C2O4���ĽṹʽΪ![]() ��Cԭ��û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ�������Ϊ�Ҽ���˫������1���Ҽ��������к���7���Ҽ���1mol���ᣨH2C2O4���к��ЦҼ�����ĿΪ7mol����3���ȵ�������ָ������ͬ�۵�����Ŀ��ԭ����Ŀ�ķ��ӻ����ӣ���SiF4��Ϊ�ȵ������������ΪSO42-��PO43-�ȣ�BF4����B�ļ۵��Ӷ���Ϊ
��Cԭ��û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ�������Ϊ�Ҽ���˫������1���Ҽ��������к���7���Ҽ���1mol���ᣨH2C2O4���к��ЦҼ�����ĿΪ7mol����3���ȵ�������ָ������ͬ�۵�����Ŀ��ԭ����Ŀ�ķ��ӻ����ӣ���SiF4��Ϊ�ȵ������������ΪSO42-��PO43-�ȣ�BF4����B�ļ۵��Ӷ���Ϊ![]() =4 Ϊsp3�ӻ�,��BF4-�Ŀռ乹��Ϊ�������壻��3��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ�����ܽ���B��F֮��ĵڶ�����Ԫ����Be��C��N��O��������4�֣���3���辧����Ni2����Ni3���ĸ����ֱ�Ϊx��y������NixO�л��ϼ۴�����Ϊ���֪2x��3y��2��x��y��0.93�����x��0.75��y��0.14����ѧʽΪNi0.75Ni0.14O��
=4 Ϊsp3�ӻ�,��BF4-�Ŀռ乹��Ϊ�������壻��3��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ�����ܽ���B��F֮��ĵڶ�����Ԫ����Be��C��N��O��������4�֣���3���辧����Ni2����Ni3���ĸ����ֱ�Ϊx��y������NixO�л��ϼ۴�����Ϊ���֪2x��3y��2��x��y��0.93�����x��0.75��y��0.14����ѧʽΪNi0.75Ni0.14O��