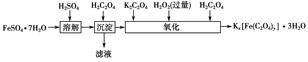
��Ŀ����
���������ؾ���Kx[Fe��C2O4��y]��3H2O��������Ӱ����ɫӡˢ��ʵ�����Ʊ����������ؾ����ʵ���������£�
��1����������ϡ�����Ʊ�FeSO4��7H2O��������______������������___________________________________________________��
��2�����������У���������һ������FeC2O4��2H2O��������ˮϴ�ӡ���������Ƿ�ϴ�Ӹɾ��ķ�����_________________________________________________________________��
��3���ⶨ���������ز�Ʒ��Fe3��������C2O42-������ʵ�鲽�����£�
����1��ȷ��ȡ���Ʋ��������ؾ���a g��Լ1.5 g�������250 mL����Һ��
����2������Һ����ȡ25.00 mL����Һ����ƿ�У�����6 mol��L��1 HCl 10 mL��������70��80 �棬������SnCl2TiCl3���ϻ�ԭ����Fe3��ȫ����ԭΪFe2��������MnSO4��Һ10 mL����75��80 ������0.010 00 mol��L��1 KMnO4����Һ�ζ����յ㣨Cl�������뷴Ӧ������C2O42-ȫ��������CO2��Fe2��ȫ��������Fe3����¼�����
����3������
����4���ظ���������2������3���Ρ�
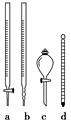
�ٲ���2����ʱ������Ҫʹ����ͼ��ʾ�����е�________������ţ���
�ڲ���2��MnSO4��Һ��������________���ζ��յ��������______________________________________��
���ڲ������Լ������ǰ���£�����3��Ŀ����_________________________��

��1����������ϡ�����Ʊ�FeSO4��7H2O��������______������������___________________________________________________��
��2�����������У���������һ������FeC2O4��2H2O��������ˮϴ�ӡ���������Ƿ�ϴ�Ӹɾ��ķ�����_________________________________________________________________��
��3���ⶨ���������ز�Ʒ��Fe3��������C2O42-������ʵ�鲽�����£�

����1��ȷ��ȡ���Ʋ��������ؾ���a g��Լ1.5 g�������250 mL����Һ��
����2������Һ����ȡ25.00 mL����Һ����ƿ�У�����6 mol��L��1 HCl 10 mL��������70��80 �棬������SnCl2TiCl3���ϻ�ԭ����Fe3��ȫ����ԭΪFe2��������MnSO4��Һ10 mL����75��80 ������0.010 00 mol��L��1 KMnO4����Һ�ζ����յ㣨Cl�������뷴Ӧ������C2O42-ȫ��������CO2��Fe2��ȫ��������Fe3����¼�����
����3������
����4���ظ���������2������3���Ρ�
�ٲ���2����ʱ������Ҫʹ����ͼ��ʾ�����е�________������ţ���
�ڲ���2��MnSO4��Һ��������________���ζ��յ��������______________________________________��
���ڲ������Լ������ǰ���£�����3��Ŀ����_________________________��
��1��������ֹFe2������������
��2��ȡ�������һ��ϴ��Һ������BaCl2��Һ�������ְ�ɫ������˵������û��ϴ�Ӹɾ�����֮��������ϴ�Ӹɾ�������������Ҳ�ɣ�
��3����ad��������������Һ��Ϊdz��ɫ���Ұ���Ӳ���ɫ���۲ⶨC2O42-�ĺ���
��2��ȡ�������һ��ϴ��Һ������BaCl2��Һ�������ְ�ɫ������˵������û��ϴ�Ӹɾ�����֮��������ϴ�Ӹɾ�������������Ҳ�ɣ�
��3����ad��������������Һ��Ϊdz��ɫ���Ұ���Ӳ���ɫ���۲ⶨC2O42-�ĺ���
��1������ϡ�����Ʊ�FeSO4��7H2O������һ�����ʹ�������Ҫ�����ᣬ��Ϊ�ڻ�þ���Ĺ���������Fe2����ˮ�⡣��2������ϴ���Ƿ�ɾ�����Ҫ�dz����������Ŀ����������Ƿ���ڣ����ȷ������ʿ�����K2SO4������ͨ������SO42-����ϴ���Ƿ�ɾ�����3����ע��ⶨ������Ҫ�����¶ȣ�ѡ���¶ȼƣ�������ͨ������KMnO4�ζ���ѡ����ʽ�ζ��ܡ��ڵζ�������MnO4-����ԭΪMn2�����ȼ���Mn2�����������������ζ��յ�ʱ��KMnO4��������ʱ��Һ��dz��ɫ���Ұ�����ڲ���ɫ���۱�ʵ���Ŀ���DzⶨFe3����C2O42-�����ⶨ�����У����Ƚ�Fe3����ԭΪFe2����Ȼ������KMnO4�ζ�����Fe2����C2O42-�����ֱ��Fe2����C2O42-���Ե�����ֻ����������˻���Ҫ�ζ���Fe2����C2O42-�������֪�ζ�C2O42-�����ף���Ϊ������ȡ��Һ��ֱ�ӵζ�����Ϊ��ʱ��ΪFe3������KMnO4��Ӧ��

��ϰ��ϵ�д�
�����Ŀ


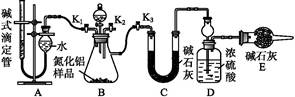


 A������
A������