��Ŀ����
(15 ��)
������ͭ�Ǻϳ��������������в��ϡ�����������ͭ����Ҫǰ����֮һ������������һ��ʵ���Һϳ�·�ߣ�

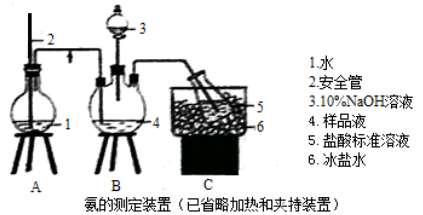
�Ʊ��������װ��ʾ��ͼ����(���Ⱥͼг�װ�õ���)��

��֪����������۵�Ϊ76.5 �棬������ˮ�������Ҵ���
�ش��������⣺
��1����250 mL����ƿa�м���70 mL70%���ᡣ���ƴ�����ʱ����������ˮ��Ũ������Ⱥ�˳����
��
��2����a�е���Һ������100 �棬�����μ�40 g�����浽������Һ�У�Ȼ��������130 �������Ӧ����װ���У�����b�������� ������c�������� ���������� ��
��Ӧ�������������ˮ���ٷ�����������Ʒ��������ˮ��Ŀ���� �����������п����ڷ��뱽�����Ʒ���� (����)��
��3���ᴿ�ֱ�����ķ����� �����յõ�44 g��Ʒ��������IJ����� ��
��4����CuCl2 ? 2H2O��NaOH��Һ�Ʊ�����Cu(OH)2�����������������ˮϴ�ӳ������жϳ���ϴ�ɾ���ʵ������������� ��
��5������������˵��Ҵ���ˮ�Ļ���ܼ��У�����ܽ����Cu(OH)2����30min�����ˣ���Һ����һ��ʱ�䣬����������ͭ���壬����ܼ����Ҵ��������� ��
������ͭ�Ǻϳ��������������в��ϡ�����������ͭ����Ҫǰ����֮һ������������һ��ʵ���Һϳ�·�ߣ�

�Ʊ��������װ��ʾ��ͼ����(���Ⱥͼг�װ�õ���)��

��֪����������۵�Ϊ76.5 �棬������ˮ�������Ҵ���
�ش��������⣺
��1����250 mL����ƿa�м���70 mL70%���ᡣ���ƴ�����ʱ����������ˮ��Ũ������Ⱥ�˳����
��
��2����a�е���Һ������100 �棬�����μ�40 g�����浽������Һ�У�Ȼ��������130 �������Ӧ����װ���У�����b�������� ������c�������� ���������� ��
��Ӧ�������������ˮ���ٷ�����������Ʒ��������ˮ��Ŀ���� �����������п����ڷ��뱽�����Ʒ���� (����)��
| A����Һ©�� | B��©�� | C���ձ� | D��ֱ��������E�������� |
��4����CuCl2 ? 2H2O��NaOH��Һ�Ʊ�����Cu(OH)2�����������������ˮϴ�ӳ������жϳ���ϴ�ɾ���ʵ������������� ��
��5������������˵��Ҵ���ˮ�Ļ���ܼ��У�����ܽ����Cu(OH)2����30min�����ˣ���Һ����һ��ʱ�䣬����������ͭ���壬����ܼ����Ҵ��������� ��
���ȼ�ˮ���ټ���Ũ����(1��)
�Ƶμӱ�����(1��)����������(1��) ����(��ʹ�����ķ�ӦҺ����)(1��)
���ڱ���������(2��) BCE(ȫѡ��2��)
���ؽᾧ(1��) 95%( 2��)
��ȡ����ϴ��Һ������ϡ���ᡢ�ټ�AgN03��Һ���ް�ɫ���dz���(2��)
�����������ܽ�ȣ����ڳ�ַ�Ӧ(2��)
���������������ˮ��Ũ������Ӧ�ȼ�ˮ���ټ���Ũ���ᣬ��ֹ���С�
������bΪ��Һ©����ͨ����Һ©��������ƿa�еμӱ����棻����cΪ���������ܣ�������������������(ʹ�����ķ�ӦҺ����)����Ӧ�������������ˮ�����ڱ�����(������ˮ)�ᾧ������ͨ�������ܴӻ��Һ�з�����������Ʒ���������õ�������Ҫ��©�������������ձ��ȡ�
�ǽ��ֱ����ᾧ����������ˮ���ܽ⣬Ȼ���ٽ��½ᾧ����(�ؽᾧ)�ɵýϴ����ı����ᾧ�壻���ݡ�1
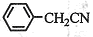 ��1
��1 ����ϵʽ���ɼ��㱽����IJ��ʣ�
����ϵʽ���ɼ��㱽����IJ��ʣ�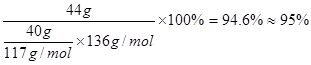 ��
�������Ʊ�Cu(OH)2������ͬʱ���п�����NaCl���ɣ����жϳ���ϴ�ɾ��ķ������Ǽ������һ��ϴ��Һ���Ƿ���Cl����
�ɸ��ݡ�������������ˮ�������Ҵ��������Ҵ���ˮ�Ļ���ܼ��е��Ҵ�������������ܽ�ȣ����ڳ�ַ�Ӧ��

��ϰ��ϵ�д�
 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ
 H2O + CH3CH2��O��CH2CH3 (����)
H2O + CH3CH2��O��CH2CH3 (����)