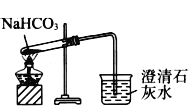
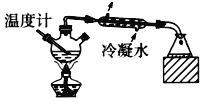
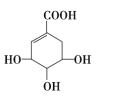
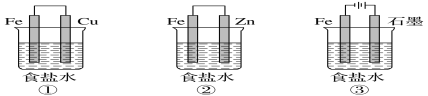
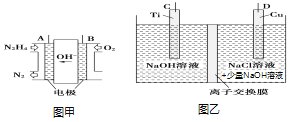
��Ŀ����
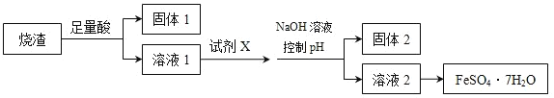
����Ŀ��A��B��C��D�����ֳ������л������A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��C��Ũ����ͼ��������·�����Ӧ�����ɵ��л�����������ζ��A��B��C��D��һ�������µ�ת����ϵ��ͼ��ʾ����Ӧ������ʡ�ԣ���
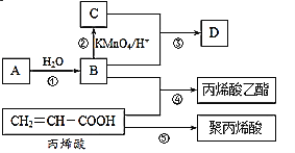
��1��C�й����ŵ�����Ϊ___���۵Ļ�ѧ����ʽΪ__��
��2����ϩ�ᣨCH2=CH��COOH�������ʿ�����___������ѡ��
A���ӳɷ�Ӧ B��ȡ����Ӧ C���кͷ�Ӧ D��������Ӧ
��3����һ�ַ�������B��C�������Լ���__��
��4����ϩ�������Ľṹ��ʽΪ___��
���𰸡��Ȼ� CH3COOH��CH3CH2OH![]() CH3COOC2H5��H2O ABCD ̼������Һ����̼��������Һ����ɫʯ��� CH2=CHCOOCH2CH3
CH3COOC2H5��H2O ABCD ̼������Һ����̼��������Һ����ɫʯ��� CH2=CHCOOCH2CH3
��������
������Ŀ��Ϣ��֪A����ϩ����ϩˮ���õ��Ҵ���BΪ�Ҵ����Ҵ���������õ����ᣬ���C�����ᣬD�Ƕ��߷���������Ӧ��õ�������������
��1������Ĺ�����Ϊ�Ȼ�������������Ӧ������ʽΪCH3COOH��CH3CH2OH![]() CH3COOC2H5��H2O��
CH3COOC2H5��H2O��
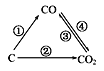
��2��̼̼˫�����Է����ӳɷ�Ӧ��������Ӧ���Ȼ����Է����кͷ�Ӧ�������ϵ���ԭ�ӿ��Ա�ȡ������˴�ѡABCD��
��3���Ҵ������ԣ������������ԣ�������̼������Һ��ʯ����Һ�ȷ���������
��4����ϩ����������ϩ����Ҵ��γɵ�������ṹΪCH2=CHCOOCH2CH3��

 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�����Ŀ���밴Ҫ��ش��������⣺
(1)25 ��ʱ����ˮ�м�������̼���ƹ��壬�õ�pHΪ11����Һ����ˮ������ӷ���ʽΪ_____________����ˮ�������c(OH��)��________mol��L��1��
(2)���볣���Ǻ���������ʵ���̶�ǿ��������������֪��
��ѧʽ | ���볣��(25 ��) |
HCN | K��4.9��10��10 |
CH3COOH | K��1.8��10��5 |
H2CO3 | K1��4.3��10��7��K2��5.6��10��11 |
��25 ��ʱ���е�pH��a.NaCN��Һ��b.Na2CO3��Һ��c.CH3COONa��Һ������Һ��Ũ���ɴ�С��˳��Ϊ___________________________��(��a b c��ʾ)
����NaCN��Һ��ͨ��������CO2��������Ӧ�Ļ�ѧ����ʽΪ_________��
(3)����ʱ����100mL 0.1mol/L NH4HSO4��Һ�еμ�0.1mol/L NaOH��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��
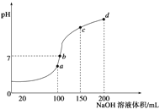
�Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶�������________����b�㣬��Һ�и�����Ũ���ɴ�С������˳����_____________________________��