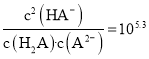��Ŀ����
����Ŀ������˵����ȷ���ǣ� ��
A.��Ϊ SO2 ����Ư���ԣ���������ʹƷ����Һ����ˮ��KMnO4(H��)��ʯ����Һ��ɫ
B.�� 50 mL 18.4 mol/L H2SO4 ��Һ�м���������ͭƬ�����ȣ���ַ�Ӧ����ԭ�� H2SO4�����ʵ���С�� 0.46 mol
C.SO2��NO2 �� CO2 ���������������Ҫԭ������ˮ�� pH С�� 5.6
D.Ũ HNO3 ��Ũ���ᰴ 3��1 ������Ȼ�����õĻ�������ˮ�����ܽ��Ͳ�
���𰸡�B
��������
A. SO2 ʹ��ˮ��KMnO4(H��)���ֵ��ǻ�ԭ�ԣ�SO2����Ư��ʯ����Һ��Aѡ�����
B. 50 mL 18.4 mol/L H2SO4��H2SO4��50��10-3L��18.4 mol/L=0.92mol������������ͭƬ�����ȣ�2H2SO4(Ũ) + Cu ![]() CuSO4 + 2H2O + SO2��������ԭ�� H2SO4ռ��Ӧ��H2SO4������һ�룬���淴Ӧ���У������ϡ����Ӧֹͣ�����ԣ���ַ�Ӧ����ԭ�� H2SO4�����ʵ���С��0.92mol��2= 0.46 mol��Bѡ����ȷ��
CuSO4 + 2H2O + SO2��������ԭ�� H2SO4ռ��Ӧ��H2SO4������һ�룬���淴Ӧ���У������ϡ����Ӧֹͣ�����ԣ���ַ�Ӧ����ԭ�� H2SO4�����ʵ���С��0.92mol��2= 0.46 mol��Bѡ����ȷ��
C. CO2 �������������ԭ��Cѡ�����
D. ��ˮ��Ũ HNO3 ��Ũ���ᰴ 1��3 ������Ȼ�����õĻ���Dѡ�����
��ѡB��

����Ŀ��������һ����Ҫ����ԭ�ϡ�
(1)�¹���ѧ�ҹ�����1902�꿪ʼ�о��ɵ���������ֱ�Ӻϳɰ���
��֪����![]() ��H=a kJ/mol
��H=a kJ/mol
��ÿ�ƻ�lmol�йػ�ѧ����Ҫ���������±���
H-H | N-H | N��N |
436kJ | 391kJ | 946k |
��a=_________________��
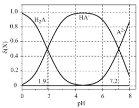
(2)��ͼΪ��ͬ�¶�(T)����ͬͶ�ϱ�[n(![]() )/n(
)/n(![]() )]ʱ��Ӧ�ﵽƽ��ʱ
)]ʱ��Ӧ�ﵽƽ��ʱ![]() ��ת���ʱ仯���ߡ�
��ת���ʱ仯���ߡ�

��![]() ��
��![]() ��
��![]() ��
��![]() �Ĵ�С��ϵΪ__________________��
�Ĵ�С��ϵΪ__________________��
�ڱ����¶Ⱥ�������䣬���Ͷ�ϱ�[n(![]() )/n(
)/n(![]() )]�������´ﵽƽ��ʱ������˵��һ����ȷ����_______(����ĸ���)��
)]�������´ﵽƽ��ʱ������˵��һ����ȷ����_______(����ĸ���)��
A.![]() ��Ũ������
��Ũ������
B.![]() ��ת��������
��ת��������
C.![]() �������������
�������������
D.![]() ��Ũ�ȼ�С
��Ũ�ȼ�С
���¶�Ϊ![]() ʱ����2L�ܱ������м���1.0mol
ʱ����2L�ܱ������м���1.0mol![]() ��1.0mol
��1.0mol![]() ����5min��Ӧ�ﵽƽ�⣬����v(
����5min��Ӧ�ﵽƽ�⣬����v(![]() )��ʾ�÷�Ӧ��ƽ������Ϊ____________________����Ӧ��
)��ʾ�÷�Ӧ��ƽ������Ϊ____________________����Ӧ��![]() ʱ��ƽ�ⳣ��K=________________��
ʱ��ƽ�ⳣ��K=________________��
(3)һ�����ͳ������е�������ķ����Dz���![]() ����ԭ����ԭ�����еĵ��������Ҳ�������Ⱦ��д��
����ԭ����ԭ�����еĵ��������Ҳ�������Ⱦ��д��![]() ��ԭ
��ԭ![]() �Ļ�ѧ��Ӧ����ʽ_____________________________________��
�Ļ�ѧ��Ӧ����ʽ_____________________________________��
(4)���ñ�����ⶨijϡ��ˮ��Ũ�ȣ�Ӧѡ��__________��ָʾ�����ζ��������۾�ע��_____________����֪ϡ��ˮ���Ϊ25.0mL���ζ������������0.0100mol/L�����ƽ�����Ϊ20.0mL����ð�ˮ��Ũ��Ϊ________________(����2λ��Ч����)�����ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������ݣ���ⶨ���________(�ƫ����ƫС���������䡱)��