��Ŀ����
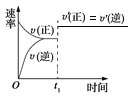
����Ŀ�����ĺϳ�ԭ��Ϊ:N2��g����3H2��g��2NH3��g��H=��92.4kJ��mol-1��500����20MPaʱ����N2��H2����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ���ش���������:
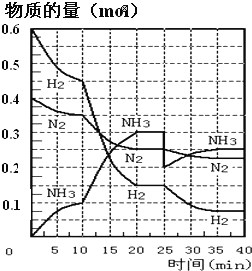
��1��10min����NH3��ʾ��ƽ����Ӧ����Ϊ_________
��2����10��20min�ڣ�NH3Ũ�ȱ仯��ԭ�������_____________
A.���˴���
B.���������
C.�����¶�
D.����NH3���ʵ���
��3����1��ƽ��:ƽ�ⳣ��K1=__________�������ݵı���ʽ������2��ƽ��ʱNH3���������Ϊ_____________��
��4�������������˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fc2O3��TiO2��������ˮ�������з�Ӧ:N2��g����3H2O��l��2NH3+1.5O2��g��H=akJ��mol-1����һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±�:
T/K | 303 | 313 | 323 |
NH3������/(10-6mol) | 4.8 | 5.9 | 6.0 |
�ٴ˺ϳɷ�Ӧ��a______0����S_______0����������������������һ����
����֪N2��g����3H2��g��2NH3��g��H=��92.4kJ��mol-1��2 H2��g���� O2��g��=2H2O��l��H=-571.kJ��mol-1 �����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽΪ_____________________________
���𰸡�0.005mol��L-1.min-1 A ![]() 45.5����0.455 �� �� 2N2(g)��6H2O(l)��4NH3(g) +3O2(g) AH����1530 kJ��mol-1
45.5����0.455 �� �� 2N2(g)��6H2O(l)��4NH3(g) +3O2(g) AH����1530 kJ��mol-1
��������
��1������v=��n��V����t���㣻
��2������ͼ��֪��ƽ��������Ӧ�����ƶ���10minʱ�������ģ������������ʵ��������ӱ�����ͬ��˵��Ϊʹ�ô�����
��3���ﵽƽ��״̬ʱ�����ʵ������䣬�Դ��жϴﵽƽ���ʱ��Σ���ѧƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ���2��ƽ��ʱNH3������������ڰ����ĺ���/ƽ��ʱ����������ʵ�����
��4�����ɱ������ݿ�֪�������¶ȣ�NH3����������˵��ƽ��������Ӧ�����ƶ�����Ϸ�Ӧ����ʽ�и����ʵľۼ�״̬���
����֪����N2��g��+3H2��g��![]() 2NH3��g����H=-92.4kJ��mol��1��2H2��g��+O2��g���T2H2O��l����H=-571.6kJ��mol��1�������ø�˹���ɣ�������2-����3�ɵó����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽ��
2NH3��g����H=-92.4kJ��mol��1��2H2��g��+O2��g���T2H2O��l����H=-571.6kJ��mol��1�������ø�˹���ɣ�������2-����3�ɵó����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽ��
��1����Ӧ����v��NH3��=(0.1mol-0)��2L��10min=0.005mol/��L��min����
��2����ͼ���֪��������ʵ����仯���ӣ���10minʱ�仯�������ģ�20min��ƽ��ʱ����n��N2��=0.025mol��4=0.1mol����n��H2��=0.025mol��12=0.3mol����n��NH3��=0.025mol��8=0.2mol�����ʵ����仯֮�ȵ��ڻ�ѧ������֮�ȣ������������ʵ��������ӱ�����ͬ��˵��10min���ܸı��������ʹ�ô�������С����൱������ѹǿ��Ӧ�÷�Ӧ����������ӱ��������¶ȣ�Ӧ�÷�Ӧ���ʼ�С������NH3���ʵ������淴Ӧ�������ӵı�����ֻ��ʹ�ô������ϣ�
��ѡA��
��3����ͼ����Կ���������Ӧ���е�ʱ20-25min�������ʵ������䣬˵����Ӧ�ﵽƽ��״̬����ѧƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ���ͼ���֪��20min��ƽ��ʱ��n��N2��=0.025mol��10=0.25mol��n��H2��=0.025mol��6=0.15mol��n��NH3��=0.025mol��12=0.3mol������������ƽ�ⳣ��K=c2(NH3)/c(N2)c3(H2)=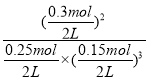 =
=![]() ��
��
��2��ƽ��ʱNH3���������=2.5mol����2.5mol+2.25mol+0.75mol����100%=45.5%��
��4�����ɱ������ݿ�֪�������¶ȣ�NH3����������˵��ƽ��������Ӧ�����ƶ���������ӦӦΪ���ȷ�Ӧ��a��0���ɷ���ʽ��֪��Ӧ������������ʵ������࣬���S��0��
����֪����N2��g��+3H2��g��![]() 2NH3��g����H=-92.4kJ��mol��1��2H2��g��+O2��g���T2H2O��l����H=-571.6kJ��mol��1
2NH3��g����H=-92.4kJ��mol��1��2H2��g��+O2��g���T2H2O��l����H=-571.6kJ��mol��1
�����ø�˹���ɣ�������2-����3�ɵó����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽΪ2N2��g��+6H2O��l��=4NH3��g��+3O2��g����H=2����-92.4kJ��mol��1��-3����-571.6kJ��mol��1��=+1530kJ��mol��1��